My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.28 n.1 Madrid Jan./Feb. 2013
https://dx.doi.org/10.3305/nh.2013.28.1.6243
ORIGINAL
Citrullinemia stimulation test in the evaluation of the intestinal function
Citrulinemia prueba de estimulación en la evaluación de la función intestinal
Beatriz Pinto Costa1, Marco Serôdio2, Marta Simões3, Caria Veríssimo3, F. Castro Sousa1 and Manuela Grazina4
1Coimbra University Medical School and IIIrd Surgical Department of Coimbra University Hospitals.
2IIIrd Surgical Department of Coimbra University Hospitals.
3Center for Neurosciences and Cellular Biology of Coimbra University
4Coimbra University Medical School and Center for Neurosciences and Cellular Biology of Coimbra University
ABSTRACT
Background: Citrullinemia is been reported as a quantitative parameter of the enterocyte mass and function.
Aim: The objective of this research is to analyse the value of fasting and stimulated citrullinemias in the intestinal function evaluation.
Methods: A case-control study was undertaken, including 11 patients with short bowel syndrome, 13 patients submitted to malabsorptive bariatric surgery and 11 healthy controls. Plasma levels of amino acids were determined, before and after a stimulation test with oral L-glutamine, by ion exchange chromatography.
Results: Citrullinemia was inferior in short bowel patients (28,6 ± 11,3 versus 35,5 ± 11 in operated obese versus 32,2 ± 6,6 μmol/L in controls; n.s.) and lower than 25,5 μmol/L in 54,5% of them (versus 16,7%; p = 0,041; accuracy = 74%; odds ratio = 3, 95%CI 1,2-7,6). ΔCitrullinemia80 (relative variation of citrullinemia at the 80th minute of test) was lower in short bowel patients; its diagnostic accuracy was similar to baseline citrullinemia and also not significant. ΔCitrullinemia80 revealed a high predictive capacity of a short bowel inferior or equal to 50 cm (auR.O.C. = 82,3%; 95%CI 61,7-102,8; p = 0,038).
Conclusions: In short bowel syndrome context, citrullinemia stimulation test with oral L-glutamine is feasible and it may improve the predictive capacity of severity. Further investigation is required to determine its clinical relevance and applicability.
Key words: Citrullinemia. Intestinal function. Short bowel syndrome. Bariatric surgery. L-glutamine.
RESUMEN
Introducción: Citrulinemia sí ha reportado como un parámetro cuantitativo de la masa y la función del enterocito.
Objetivo: El objetivo de esta investigación es analizar el valor de las citrulinemias en ayuno y estimulada en la evaluación de la función intestinal.
Métodos: Un estudio de casos y controles se llevó a cabo, incluyendo 11 enfermos con síndrome del intestino corto, 13 pacientes sometidos a cirugía bariátrica de malabsorción y 11 controles sanos. Los niveles plasmáticos de aminoácidos se determinaron, antes y después de la prueba de estimulación oral con L-glutamina, por cromatografía de intercambio iónico.
Resultados: Citrulinemia fue menor en los pacientes de intestino corto (28,6 ± 11,3 versus 35,5 ± 11 en los obesos operados versus 32,2 ± 6,6 μmol/L en los controles; n.s.) e inferior a 25,5 μmol/L en el 54,5% de ellos (versus 16,7%; p = 0,041, exactitud = 74%, odds ratio = 3, IC95% 1,2 a 7,6). ΔCitrullinemia80 (variación relativa de la citrulinemia a los 80 minutos de la prueba) fue menor en enfermos de intestino corto; su precisión diagnóstica fue similar a la citrulinemia en ayuno y también no significativa. ΔCitrullinemia80 reveló una elevada capacidad predictiva de intestino corto inferior o igual a 50 cm (abR.O.C. = 82,3%; IC95% 61,7-102,8; p = 0,038).
Conclusiones: En el contexto de lo síndrome de intestino corto, la prueba de estimulación de la citrulinemia con L-glutamina oral es factible y puede mejorar la capacidad predictiva de gravedad. Se requieren nuevas investigaciones para determinar su importancia clínica y aplicabilidad.
Palabras clave: Citrulinemia. Función intestinal. Síndrome de intestino corto. Cirugía bariátrica. L-glutamina.
Abbreviations
BMI: Body mass index.
auROC: Area under the "receiver operating characteristic curve".
95%CI: 95% Confidence interval.
n.s.: statistically not significant.
vs: versus.
Introduction
Several authors suggest that citrullinemia may constitute an objective, quantitative, reproducible and simple parameter of the functional enterocyte mass, independent of the nutritional status, the presence of local inflammation and the etiology of the lesion, in different ages and pathologies (such as short bowel syndrome, villous atrophy, radio and chemotherapy enteropathies and acute rejection of small bowel transplant)1-3.
Indeed, in the human, citrulline is a non-protein amino acid that results from the enterocyte mitochondrial metabolism of glutamine, particularly in the proximal small bowel, at the upper and medium part of the villi1,2,4. After synthesis, regulated by pyrroline 5-carboxylate synthase, an enzyme almost exclusive of the enterocytes, citrulline is released in the portal circulation and converted to arginine in the kidneys1,2,4-6. The intestine represents the main source of circulating citrulline1,2,4-6.
Various studies demonstrated that citrullinemia is correlated with intestinal length, mass and absorptive function, in adults and children1,2,7. In short bowel syndrome, it seems to represent an important predictive factor of irreversible intestinal failure and a parameter for monitorization of the physiological adaptation and the surgical rehabilitation1,2,8-10.
Despite favorable reports associating fasting citrullinemia to the degree of functional enterocyte mass reduction in various small bowel disorders, some limitations have been highlighted. Several authors1,11-13 emphasized the inconsistent correlation between citrullinemia and intestinal absorption of macro and micronutrients, even in cases of successful dietetic and pharmacological rehabilitation; although absorption constitutes a complex integrated process influenced by other factors (as biliopancreatic secretions, digestive motility and colonic mucosa), various methodological aspects of those studies might have interfere with the conclusions and citrulline remains regarded as an indicator of the integrity and functionality of the enterocytes1, specially of duodenum and jejunum. Precise determination of diagnostic and prognostic thresholds of citrullinemia is also required owing to the overlapping of values with apparently different clinical significances8,10. Furthermore, plasma citrulline concentrations seem to reflect predominantly the extremes of the disease spectrum and, in cases of intermediate severity as those of small bowel syndrome with residual intestine between 50 and 150 cm, fasting citrullinemia may be insufficiently discriminative for use in the individual context1,10,11. A dynamic evaluation of the citrullinemia production, using a stimulation test with exogenous glutamine, may improve the discriminative accuracy and overcome some of the referred limitations.
Objectives
The objective of present study is to determine the value of fasting citrullinemia and citrullinemia stimulation test in the intestinal function evaluation.
Material and methods
This study included adult patients with short bowel syndrome (defined as a postduodenal small bowel remnant length inferior to 200 cm10,14) consequent to massive enterectomy; patients subjected to malabsorptive bariatric surgery (including gastric by-pass and duodenal switch), with a follow-up period of at least six months and a control group of healthy individuals, with 18 to 75 years-old, body mass index between 18,5 and 35 Kg/m2, stable body weight in the last six months (variation inferior to 5%) and without exclusion criteria. The exclusion criteria included urea cycle or citrulline metabolism disorders, renal insufficiency (creatininemia > 1,8 mg/dL), previous hepatic or pancreatic surgery, pregnancy and lactation, in all groups; known digestive disease, previous digestive surgery (except appendicectomy), uncontrolled diabetes mellitus, autoimmune disease, acquired immunodeficiency syndrome, significant organ insufficiency, severe metabolic stress, use of glucocorticoids, intestinal transit modulators or microbiotics administration, medium chain triglycerides, glutamine or citrulline supplementation in the previous month, in control group.
In short bowel patients, the etiology, anatomic type, residual bowel characteristics (segment, length, integrity and transit continuity), evolution phase, adaptation grade (characterized by the degree and duration on nutritional support dependence10,14) and the eventual intestinal rehabilitation therapies were recorded. Length of residual post-duodenal small bowel was measured peroperatively at the antimesenteric border. Actual status of primary disease (active versus controlled), digestive symptoms, associated diseases (including liver or pancreatic dysfunction) and medications were also registered.
In obese patients' group, gastric by-pass was accomplished by laparoscopic approach and involved a restriction gastroplasty associated to a jejunal transsection 100 cm distal to the Treitz's angle and a Roux-en-Y gastroenterostomy, creating an alimentary segment with 150 cm and a biliopancreatic branch with 100 cm of length. Duodenal switch was performed by laparotomy and included a longitudinal gastrectomy of the greater curvature, the closure of the duodenum, an intestinal transsection 250 cm proximal to the ileocecal valve and a Roux-en-Y duodenumileostomy, with an entero-enterostomy 100 cm proximal to the ileocecal valve, creating an alimentary segment with 150 cm and a common branch (alimentary and biliopancreatic) with 100 cm of length. In patients subjected to bariatric surgery, the preoperative and actual weights, type of surgical technique, follow-up time, comorbidities (such as dyslipidemia, diabetes mellitus, arterial hypertension, obstructive sleep apnea, psychiatric disorders and osteoarticular disease) and its evolution (noticing as favorable outcome the restoration of laboratory values and/or the diminution or suspension of the medical therapy) were registered. Digestive symptoms, concomitant diseases (hepatic or pancreatic insufficiency and others) and medications were also noticed.
Blood was collected, after an eight hours fasting period, for determination of amino acid plasma levels (citrulline, glutamine, arginine, ornithine, alanine, isoleucine, proline and glutamic acid) and regular analysis (including serum biochemistry with liver enzymes, ionograme, creatinine, ureic nitrogen and lipids profile; hemograme with leucocyte formula; caolin-cefalin and prothombine times and C-reactive protein). Plasma aminogram was repeated 80 and 120 minutes after the stimulation test that included an oral bolus administration of a L-glutamine solution (0,2 g/Kg) as Glutamine Plus Orange® (Fresenius Kabi, Germany); additional oral ingestion of liquids or solids was forbidden during the test. Each sachet of Glutamine Plus Orange® was diluted in 200 ml of water and contained 10 g of glutamine, 9,4 g of maltodextrine and starch, 1 g of fibers, 1,6 mg of β-carotene, 83 mg of vitamin E, 250 mg of vitamin C, 6 mg of sodium, 55 mg of potassium, 3,3 mg of zinc and 50 μg of selenium.
Plasma concentrations of amino acids were studied by ion exchange chromatography in a high pressure system (Biochrom 30 analyzer). Plasma was extracted from blood sampled in ethilenediaminotetraacetic acid and reserved at 4oC, by centrifugation at 4000 g, 4oC, during 10 minutes; samples were prepared with ditioteitol 12%, five to 10 minutes, deproteinized with sulfosalicilic acid, 60 minutes at room temperature and, after division of the sediment by centrifugation, were filtered and preserved at -20oC for subsequent analysis. Relative variation of aminoacidemia between the baseline level and the concentration eighty minutes after the intake of L-glutamine, designated by Δaminoacidemia80, was expressed in percentage and was calculated in accordance to the formula: AAminoacidemia80 = (Eighty minute aminoacidemia/Baseline aminoacidemia) x 100 - 100. Creatinine clearance was valued through the Cockcroft e Gault formula15 based on creatininemia (determined by isotopic dilution mass spectrometry).
Nutrition status was evaluated by anthropometric (actual and usual body weights, height, triceps skinfold thickness and mid-arm circumference)16-18 and laboratorial (albuminemia) criteria. Anthropometric parameters were determined and valued in consonance to the reference tables (standardized for age and sex) and the Garrow s, McWhirter s and Blackburn's criteria16-18. Height and body weight were measured in upright position with a stadiometer and an electronic scale (Seca 644; Seca, Ltd; Germany) and were registered to the nearest 0,1 cm and 0,1 Kg, respectively. Triceps skinfold thickness corresponds to the mean of three consecutive measurements with a skinfold caliper (Holtain Ltd, Crymych; United Kingdom; 0,2 mm) applied at the back of the non-dominant arm, at the midpoint between the tip of the acromial process of the scapula and the olecranon process of the ulna, three seconds after its application. Mid-arm circumference was measured using a non-stretchable flexible tape, perpendicularly to the long axis of the arm, at same site and position as described for triceps skinfold thickness, in triplicate, to the nearest 0,1 cm16. Ideal weight was estimated with formulas based on the tables of standardized weight and height and the percentage of excess of weight loss was calculated in concordance with Deitel M et al19.
Body composition was assessed by single frequency bioelectrical impedance analysis, with determination of the right hand-to-foot resistance at 50 KHz (Bodys-tat 1500; Bodystat Ltd; British Isles)20.
Data management and statistical analysis were performed with SPSS Software version 15 for Windows (SPSS Inc., Chicago, IL), including Qui-square and Student's t tests, Anova I, Pearson's correlations and Receiver Operating Characteristic (ROC) curves. Statistical significance was considered at a P value <0,05. Study was conducted in consonance to the principles of the Helsinki declaration21 and after informed consent of the participant subjects.
Results
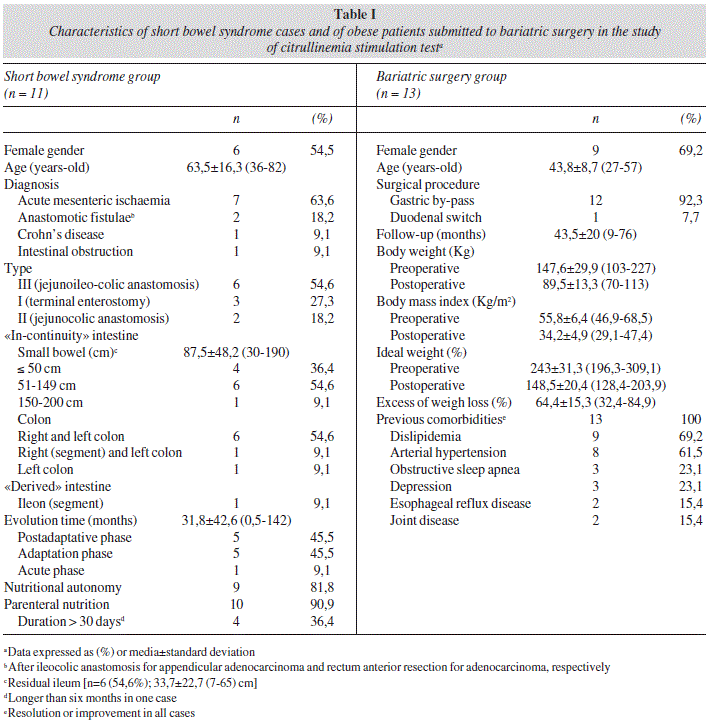
Eleven cases of short bowel syndrome with the characteristics described in table I were studied. Six patients presented a type III syndrome and five were in post-adaptation phase; ten were initially submitted to parenteral nutritional support and nine achieved nutritional autonomy. Mean length of "in-continuity" residual small bowel was 87,5 ± 48,2 (30-190) cm (inferior or equal to 50 cm in four patients). Acute mesenteric ischemia was the most frequent subjacent disease (64%). Two cases presented malnutrition (severe in one of them) according to the anthropometric criteria of McWhirter, Blackburn and Garrow and ten (91%) revealed a free-fat mass percentage below the recommended values. Five patients (45,5%) referred diarrhea, defined as daily fecal output superior to 200 g. Hypomagnesaemia was the most frequent electrolytic disturbance (54,6%).
Thirteen patients subjected to bariatric surgical procedures (12 gastric by-pass and one duodenal switch) were included, with a mean follow-up of 43,5 ± 20 (9-76) months (table I). Postoperative excess of weight mean loss was 64,4 ± 15,3 (32,4-84,9)%. Obese patients presented a mean actual body mass index of 34,2 ± 4,9 kg/m2 and an excess of body weight of 48,5 ± 20,4%. All operated obese showed amelioration or remission of the comorbidities.
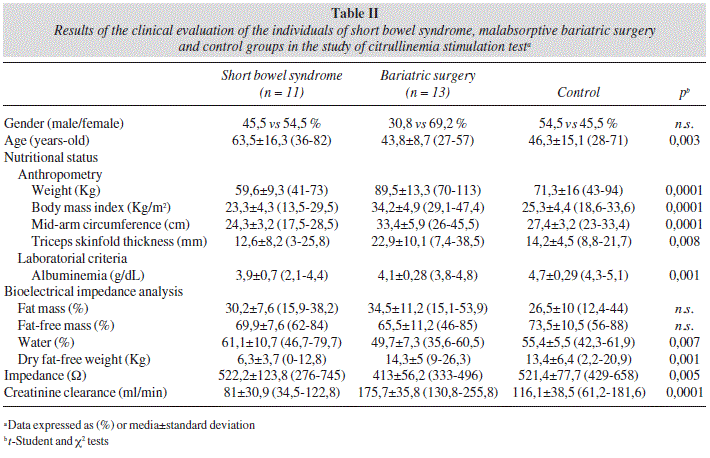
Studied groups presented significant differences in the mean values of age, anthropometric parameters, albuminemia, percentage of corporal water and dry fat-free weight (bioelectric impedance analysis) and creatinine clearance (table II). Short bowel patients demonstrated older mean age and lower mean creatinine clearance than control individuals.
Mean citrullinemia in patients with short bowel syndrome was 28,6 ± 11,3 (11-49) μmol/L [versus 35,5 ± 11,1 (20-56) μmol/L in operated obese versus 32,2 ± 6,6 (19-42) μmol/L in controls; n.s.] (fig. 1) and was less than 25,5 mol/L in 54,5% of them [versus 16,7% in the others; p = 0,041; sensitivity = 54,6%; specificity = 83,3%; accuracy = 74,3%; negative predictive value = 80%; positive predictive value = 60%; odds ratio = 3 (95%CI 1,2-7,6)]. Citrullinemia predictive capacity of short bowel syndrome was low and not significant [auROC = 66,7% (95%CI 45,5-87,8)]. Probability of a short bowel syndrome, calculated by the logistic regression model, was inversely related with citrullinemia: 23,4 ± 7,1% (95%CI 20,2-26,6) when superior than 30 pmol/L, 41 ± 3,5% (95%CI 38,7-43,4) between 20 and 30 pmol/L and 52,3 ± 7,4% (95%CI 34,1-70,6) when inferior than 20 pmol/L (p = 0,0001). Nevertheless, patients with bowel length below 50 cm presented with higher mean age (78 ± 5,7 versus 47,2 ± 12,9; p = 0,0001) and lower creatinine clearance (56,8 ± 9,8 versus 136,3 ± 49,2; p = 0,0001); two of them (50%), older than 70 years-old and with creatinine clearance below 60 ml/min, demonstrated citrullinemias of 46 and 49 μmol/L, respectively. The lowest citrulline plasma concentration (11 μmol/L) was observed in a 82 years-old female patient presenting a type III short bowel syndrome with 50 centimeters of residual intestine and submitted to an extended enterectomy, two weeks before, motivated by an acute mesenteric ischemia. Lowest citrullinemia in the obese group occurred after duodenal switch (n.s.); citrulline plasma concentrations that exceed the superior reference limit of the laboratory (43 μmol/L) were observed in four obese patients.
In the three groups, citrullinemia didn't correlate with the studied parameters of nutritional evaluation and body composition.
Plasma levels of ornithine, amino acid precursor of citrulline, were lower in short bowel patients [68,8 ± 24 (36-104) μmol/L versus 74 ± 24,8 (48-141) μmol/L in operated obese versus 94,6 ± 17,9 (52-114) μmol/L in controls; p = 0,026] (fig. 1) and inferior to 51,5 mol/L in 66,7% of those cases (versus 33,3%; w.s.).
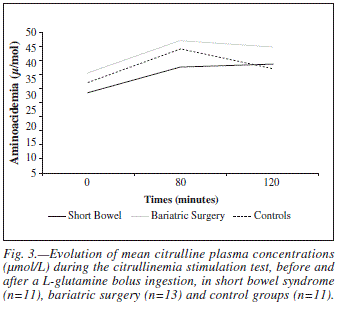
In the citrullinemia stimulation test, eighty minutes after the bolus ingestion of L-glutamine, an increase of plasma concentrations of the analyzed amino acids was observed, except of leucine and isoleucine in all groups, proline in obese and controls and ornithine in controls (fig. 2). In healthy individuals, after eighty minutes, mean increases of citrullinemia and glutaminemia were 38,9 ± 34% (p = 0,005) and 52,9 ± 22,4% (p = 0,0001), respectively (fig. 2). In short bowel patients, an attenuated and delayed citrulline response to oral L-glutamine was verified, with lower and later peak concentrations; in obese, the reduction of citrullinemia after the 80th minute was slower and attenuated (fig. 3).
Mean ΔCitrullinemia80 values were inferior in short bowel patients (33,8 ± 58,8 versus 36,9 ± 28,1%; n.s.) (fig. 2) but its diagnostic accuracy was lower than that of baseline citrullinemia and also not significant [auR.O.C. = 54,5% (95%CI 30,4-78,7), n.s. versus 66,7% (95%CI 45,5-87,8), n.s., respectively]. Mean ΔCitrullinemia80 values inferior to 8,75% were observed in 36,4% of short bowel patients [versus 4,2% in the others; p = 0,026; sensitivity = 36,4%; specificity = 95,8%; accuracy = 77,1%; negative predictive value = 76,7%; positive predictive value = 80%; odds ratio = 3,1 (95%CI 1,3-137,7)]. ΔCitrullinemia80 revealed a significant and high predictive capacity of a short bowel remnant inferior or equal to 50 cm (auR.O.C. = 82,3%, 95%CI 61,7-102,8, p = 0,038); mean values lower to 31,5% were observed in all of those patients (versus 45,2%, n.s.). Probability of a short bowel inferior or equal to 50 cm, calculated by the logistic regression model, was inversely and significantly related with Acitrullinemia80: 55,5 ± 32,1% (95%CI 4,4-106,6) when lower than 0%, 10,9 ± 3,2% (95%CI 9,1-12,8) between 0 and 31,5% and 1,5 ± 1,5% (95%CI 0,7-2,3) when higher than 31,5% (p = 0,0001). Δcitrullinemia80 didn't correlated significantly with the analyzed nutritional and body composition parameters except with the body water percentage (Pearson's coefficient = 34,9%; p = 0,04).
Discussion
In this study, according to the literature1, short bowel syndrome cases presented, in comparison with controls, lower plasma concentrations of citrulline (n.s.), arginine (n.s.) and ornithine (p = 0,026) and higher levels of glutamine (n.s.).
Mean values of citrullinemia were lower in short bowel patients (although with a statistically not significant difference) and inferior to 25,5 mol/L in 54,5% of those cases (versus 16,7%; p = 0,041). This threshold, although with low sensitivity, was associated with high specificity and negative predictive values and was similar to those described in others series1. According to Crenn P et a!10, a citrullinemia lower than 30 pmol/L, in adults, is associated with short bowel syndrome with a sensitivity of 77% and a specificity of 75% (diagnosis) and a concentration below 20 pmol/L determines the definitive character of the intestinal failure (prognosis) with a sensitivity and a specificity of 92 and 90%, respectively.
Significantly older age and lower creatinine clearance of short bowel syndrome patients when compared to controls might have contributed to minimize the difference between the citrulline plasma levels of both groups; because those factors are generally associated with elevation of citrullinemia1. Furthermore, the small number of short bowel patients included in this study, 63,7% with more than 50 cm of remnant intestine and 82% with oral nutritional autonomy, might have contributed for the absence of a significant difference between mean citrullinemias of the two groups. Unexpected high citrullinemia in two elderly patients with less than 50 cm of residual bowel, probably related with the deterioration of renal function, contributed to the absence of a statistically significant relation between citrullinemia and this pejorative prognostic factor.
In this series, the relative reduction of mean citrullinemia values in short bowel patients seems to be independent of the nutritional status, as those weren't significantly related with the evaluated nutritional and body composition criteria; those results were concordant with the literature1 and represent an advantage of citrullinemia as a parameter of evaluation of the intestinal function.
Morbid obese patients included in the current series underwent bariatric procedures with a simultaneous malabsorptive and restrictive character and a potential influence to reduce citrullinemia derived, among other factors, from the exclusion of the proximal 100 to 150 cm of the jejunum. Postoperative mean loss of excess of weight [62,7 ± 14,6 (32,4-83) after gastric by-pass and 84,9% after duodenal switch] was similar to the data published in the literature22,23.
Mean values of fasting citrullinemia in patients submitted to bariatric surgery were within the reference range of healthy occidental individuals [40 ± 10 (20-60) mol/L]1 and were analogous to those observed in controls. Those results might be attributed to a non-declared low protein diet, an inadequate acuity of citrullinemia to detect the malabsorptive consequences of bariatric surgery and to the fact that citrullinemia may reflect the global intestinal function, including that of the segments excluded of the digestive circuit. In omnivorous animals, the intestine-kidney citrulline-arginine metabolism seems to represent a process of fast adaptation to the variations of protein ingestion, with preference for the citrulline pathway in low protein diets (in order to reduce the liver uptake of arginine and the ureagenesis)1. Morimoto BH et al24 also demonstrated that citrulline production by the gut increased, by disinhibition of ornithine carbamoyltransferase, when the protein supply was low. In our study, citrullinemia exceed, unexpectedly, the reference limit in four obese operated patients; moreover, the different responses of plasma levels of arginine and ornithine to the stimulation test in obese group and controls also reinforce this low protein diet theory.
Globally, none of the studied individuals demonstrated clinical manifestations suggestive of an eventual and inherited enzymatic disease related with the Krebs-Henseleit cycle, like ornithine transcarbamoylase deficiency or citrullinemia (consequent to a disorder of the argininosuccinate synthase activity), susceptible to influence citrulline plasma levels4.
In 2007, Papadia C et al8 demonstrated a quadratic (and not linear) correlation, positive, strong and statistically significant, between citrullinemia and intestinal length; concentrations higher than 23 μmol/L were associated to normal length, lower than 21 μmol/L suggested a dependence on parenteral nutritional and below 12 μmol/L indicated an intestine with less than 50 cm; but, the positive relationship between citrullinemia and small bowel length was attenuated for higher citrulline values. Clinical relevance of citrullinemia would be greater in those patients with intermediate remnant small bowel length (50 to 150 cm), more challenging in terms of defining prognosis, namely in predicting the irreversible intestinal failure and monitoring the rehabilitation treatment.
In order to determine the potential clinical interest of a dynamic evaluation of citrullinemia, to improve its diagnostic and prognostic discriminative accuracies, a stimulation test with exogenous glutamine was performed in this study. In the enterocyte, citrulline is synthesized from glutamine, that constitutes more than 80% of its precursors and was obtained, in a considerable proportion (66%) from the intestinal lumen2,11; in 2008, Peter JHC et al11 found, in 19 healthy individuals, that an oral bolus of alanine-glutamine dipeptide causes a time-dependent rise of citrullinemia up to a peak concentration after 77 ± 16 minutes. So, an oral intake of glutamine might increase citrulline output to an extent reflecting the enterocyte functional capacity. Those were the basis for introducing a new modified stimulation test for intestinal function evaluation, with determination of citrullinemia 80 minutes after the administration of L-glutamine, in a weight-adapted dose and an oral formulation.
In fact, in present series, oral L-glutamine, although its relative instability and low solubility in water11 triggers a significant initial increment of glutamine and citrulline plasma levels in healthy controls followed, after eighty minutes, by a decrease, perhaps associated with the conversion of citrulline into arginine. A close correlation between glutamine uptake and citrulline release by the gut has been demonstrated in adults25; glutamine seems to activate argininosuccinate synthase and thus to favor recycling of citrulline into arginine26. In short bowel syndrome patients, an expected attenuated and delayed citrulline response to the oral L-glutamine was verified.
Although mean ΔCitrullinemia80 values were inferior in short bowel patients (33,8 ± 58,8 versus 36,9 ± 28,1%; n.s.), especially in cases with unfavorable prognostic factors such as an intestine remnant shorter than 50 centimeters, ACitrullinemia80 diagnostic accuracy for short bowel syndrome was lower than that of baseline citrullinemia and also not significant. Nevertheless, ΔCitrullinemia80 revealed a significant and high predictive capacity of a short bowel remnant inferior or equal to 50 cm.
The stimulation test reflects predominantly the function of proximal small bowel since glutamine, as virtually all amino acids, is absorbed in the first 100 to 150 cm of intestine and jejunum represents the main site of production of citrulline1,11; this might constitute a limitation of the test as the intestinal adaptation process to short bowel syndrome occurs principally in the ileum.
In the stimulation test, a pronounced variability of the results of citrullinemia analysis was evident, even within the three analyzed groups. Plasma concentrations of amino acids were determinate by ion exchange chromatography which is considered the reference method and allows a rapid and fully automatized assay; however, the use of reversed liquid phase chromatography, associated with higher sensitivity (although with lower reproducibility) might be more advantageous for this sequential analysis1,2,4.
Finally, the potential relevance of ornithine, an immediate precursor of citrulline in the urea cycle1,4, in the intestinal function evaluation is difficult to anticipate and justifies further investigation.
The principal limitation of this study was the low dimension of the sample, similarly to many other published series concerning this subject; it may have contributed to the apparently disappointing accuracy of fasting and stimulated citrullinemias. Preponderance of short bowel patients with favorable prognosis, particularly with oral nutritional autonomy and type III syndrome and the heterogeneity of the cases also concurred to difficult the analysis of our results. Nevertheless, those results seem to be concordant with the interest of citrullinemia as a promising parameter for the evaluation of intestinal function.
Conclusions
Present preliminary analysis suggests that citrullinemia, although susceptible to clinical and analytical interferences, may be useful in short bowel syndrome for prognosis definition and monitorization.
In this study, citrullinemia stimulation test with oral L-glutamine was feasible and, although its diagnostic accuracy seems to be similar to fasting citrullinemia in short bowel syndrome context, it may improve the predictive capacity of severity. Further investigation is required to determine its clinical relevance and applicability.
Authors' contributions
BPC: Conception and design of the study; acquisition, analysis and interpretation of data and writing the article. MS: Acquisition of data and revision of the article. MS: Acquisition of data and revision of the article. CV: Acquisition of data and revision of the article. MG: Acquisition, analysis and interpretation of data; writing and revising the article. FCS: Analysis and interpretation of data; drafting and revising the article. All authors: Reading and approval of the final version of the manuscript.
References
1. Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 2008; 27: 328-39. [ Links ]
2. Curis E, Crenn P, Cynober L. Citrulline and the gut. Curr Opin Clin Nutr Metab Care 2007; 10: 620-6. [ Links ]
3. Blasco Alonso J, Serrano Nieto J, Navas López VM, Barco Gálvez A, Vicioso I, Carazo Gallego B, Ortiz Pérez P, Sierra Salinas C: Plasma citrulline as a marker of loss of enterocitary mass in coeliac disease in childhood. Nutr Hosp 2011; 26:807-13. [ Links ]
4. Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S, Cynober L. Almost all about citrulline in mammals. Amino Acids 2005; 29: 177-205. [ Links ]
5. Bertholo RF, Burrin DG. Comparative aspects of tissue glutamine and proline metabolism. J Nutr 2008; 138: 2032S-2039S. [ Links ]
6. Deutz NEP. The 2007 ESPEN Sir David Cuthbertson lecture: Amino acids between and within organs. The glutamate-glutamine-citrulline-arginine pathway. Clin Nutr 2008; 27: 321-6. [ Links ]
7. Picot D, Garin L, Trivin F, Kossovsky MP, Darmaun D, Thibault R. Plasma citrulline is a marker of absorptive small bowel length in patients with transient enterostomy and acute intestinal failure. Clin Nutr 2010; 29: 235-42. [ Links ]
8. Papadia C, Sherwood RA, Kalantzis C, Wallis K, Volta U, Fiorini E, Forbes A. Plasma citruline concentration: a reliable marker of small bowel absortive capacity independent of intestinal inflammation. Am J Gastroenterol 2007; 102: 1474-82. [ Links ]
9. Wales PW, de Silva N, Langer JC, Fecteau A. Intermediate outcomes after serial transverse enteroplasty in children with short bowel syndrome. J Pediatr Surg 2007; 42: 1804-10. [ Links ]
10. Crenn P, Condray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absortive enterocyte mass and intestinal failure in humans. Gastroenterology 2000; 119: 1496-505. [ Links ]
11. Peters JH, Wierdsma NJ, Teerlink T, van Leeuwen PA, Mulder CJ, van Bodegraven AA. The citrulline generation test: proposal for a new enterocyte function test. Aliment Pharmacol Ther 2008; 27: 1300-10. [ Links ]
12. Luo M, Fernández-Estívariz C, Manatunga AK, Bazargan N, Gu LH, Jones DP, Klapproth JM, Sitaraman SV, Leader LM, Galloway JR, Ziegler TR. Are plasma citrulline and glutamine biomarkers of intestinal absorptive function in patients with short bowel syndrome? J Parenter Enteral Nutr 2007; 31: 1-7. [ Links ]
13. Peters J, Wierdsma N, Teerlink T, van Leeuwen P, Mulder C, van Bodegraven A. Poor diagnostic accuracy of a single fasting plasma citrulline concentration to assess intestinal energy absorption capacity. Am J Gastroenterol 2007; 102: 1-6. [ Links ]
14. O'Keefe SJD, Buchman AL, Fishbein TM, Jeejeebhoy KN, Jeppesen PB, Shaffer J. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clinical Gastroenterology and Hepatology 2006; 4: 6-10. [ Links ]
15. Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31-5. [ Links ]
16. Ravasco P, Camilo ME, Gouveia-Oliveira A, Adam S, Brum G. A critical approach to nutritional assessment in critically ill patients. Clin Nutr 2002; 21: 73-7. [ Links ]
17. Heymsfield SB, Baumgartner RN, Pan SF. Nutritional assessment of malnutrition by anthropometric methods. In: Shils ME, Olson JA, Shike M, Ross AC, Eds. Modern nutrition in health and disease, 9th edn. Baltimore: Williams and Wilkins, 1998: 903-21. [ Links ]
18. Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr 1981; 34:2540-5. [ Links ]
19. Deitel M, Gawdat K, Melissar J. Reporting weight loss 2007. Obe Surg 2007; 17: 565-8. [ Links ]
20. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AM, Pichard C; Composition of the ESPEN Working Group. Bioelectrical impedance analysis - part I: review of principles and methods. Clin Nutr 2004; 23: 1226-43. [ Links ]
21. World Medical Association Declaration of Helsinki - Ethical principles for medical research involving human subjects. Accessed in December 23rd, 2008 at (World Medical Association's website): www.wma.net/e/policy/b3.htm. [ Links ]
22. Campos GM, Rabl C, Mulligan K, Posselt A, Rogers SJ, Westphalen AC, Lin F, Vittinghoff E. Factors associated with weight loss after gastric bypass. Arch Surg 2008; 143: 877-84. [ Links ]
23. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292: 1724-37. [ Links ]
24. Morimoto BH, Brady JF, Atkinson DE. Effect of level of dietary protein on arginine-stimulated citrulline synthesis. Correlation with mitochondrial N-acetylglutamate concentrations. Biochem J 1990; 272: 671-5. [ Links ]
25. Fujita T, Yanaga K. Association between glutamine extraction and release of citrulline and glycine by the human small intestine. Life Sci 2007; 80: 1846-50. [ Links ]
26. Cynober L, Moinard C, De Bandt JP. The 2009 ESPEN Sir David Cuthbertson. Citrulline: a new major signaling molecule or just another player in the pharmaconutrition game? Clin Nutr 2010; 29:545-51. [ Links ]
![]() Correspondence:
Correspondence:
Beatriz Pinto da Costa.
Clínica Universitária de Cirugia III.
Hospitais da Universidade de Coimbra.
Praceta Prof. Mota Pinto.
3000-075 Coimbra (Portugal).
E-mail: beatrizpcosta@iol.pt
Recibido: 14-X-2012.
Aceptado: 23-XI-2012.