Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.28 no.3 Madrid Mai./Jun. 2013
https://dx.doi.org/10.3305/nh.2013.28.3.6413
ORIGINAL
What are the most effective methods for assessment of nutritional status in outpatients with gastric and colorectal cancer?
¿Cuáles son los métodos más eficaces de valoración del estado nutricional en pacientes ambulatorios con cáncer gástrico y colorrectal?
Mariana Abe Vicente1, Katia Barão1, Tiago Donizetti Silva2 and Nora Manoukian Forones3
1Nutritionist. Master of Science.
2Biologist. Master of Science.
3Head of the oncology group from the Gastroenterology Division.
Oncology Group-Gastroenterology Division. Universidade Federal de São Paulo. Brazil.
This study was supported by the São Paulo Research Foundation (FAPESP, grant 10/19191-2).
ABSTRACT
Objective: To evaluate methods for the identification of nutrition risk and nutritional status in outpatients with colorectal (CRC) and gastric cancer (GC), and to compare the results to those obtained for patients already treated for these cancers.
Methods: A cross-sectional study was conducted on 137 patients: group 1 (n = 75) consisting of patients with GC or CRC, and group 2 (n = 62) consisting of patients after treatment of GC or CRC under follow up, who were tumor free for a period longer than 3 months. Nutritional status was assessed in these patients using objective methods [body mass index (BMI), phase angle, serum albumin]; nutritional screening tools [Malnutrition Universal Screening Tool (MUST), Malnutrition Screening Tool (MST), Nutritional Risk Index (NRI)], and subjective assessment [Patient-Generated Subjective Global Assessment (PG-SGA)]. The sensitivity and specificity of each method was calculated in relation to the PG-SGA used as gold standard.
Results: One hundred thirty seven patients participated in the study. Stage IV cancer patients were more common in group 1. There was no difference in BMI between groups (p = 0.67). Analysis of the association between methods of assessing nutritional status and PG-SGA showed that the nutritional screening tools provided more significant results (p < 0.05) than the objective methods in the two groups. PG-SGA detected the highest proportion of undernourished patients in group 1. The nutritional screening tools MUST, NRI and MST were more sensitive than the objective methods. Phase angle measurement was the most sensitive objective method in group 1.
Conclusion: The nutritional screening tools showed the best association with PG-SGA and were also more sensitive than the objective methods. The results suggest the combination of MUST and PG-SGA for patients with cancer before and after treatment.
Key words: Nutritional assessment. Patient Generated Subjective Global Assesment. Colorectal cancer. Gastric cancer. Outpatients.
RESUMEN
Objetivo: Evaluar los métodos para la identificación del riesgo nutricional y del estado nutricional en pacientes ambulatorios con cáncer colorrectal (CCR) y cáncer gástrico (CG) y comparar los resultados con los obtenidos por los pacientes ya tratados por estos cánceres.
Métodos: Se realizó un estudio transversal en 137 pacientes: el grupo 1 (n = 75) comprendía pacientes con CG o CCR y el grupo 2 (n = 62) comprendía pacientes tras el tratamiento de CG o CCR en seguimiento y que estaban libres de tumor por un periodo mayor de 3 meses. Se evaluó el estado nutricional de estos pacientes usando métodos objetivos [índice de masa corporal (IMC), el ángulo de fase y la albúmina sérica]; herramientas de cribado nutricional [Malnutrition Universal Screening Tool (MUST), Malnutrition Screening Tool (MST), Nutritional Risk Index (NRI)] y una evaluación subjetiva [Evaluación Global Subjetiva Generada por el Paciente (EGS-GP)]. La sensibilidad y especificidad de cada método se calcularon con relación a la EGS-GP, que se empleó como prueba de referencia.
Resultados: 137 pacientes participaron en el estudio. Los pacientes con cáncer en estadio IV fueron más frecuentes en el grupo 1. No hubo diferencias en el IMC entre los grupos (p = 0,67). El análisis de la asociación entre los métodos de evaluación nutricional y la EGS-GP mostró que las herramientas de cribado nutricional proporcionaban resultados más significativos (p < 0,05) que los métodos objetivos en ambos grupos. La EGS-GP detectó la mayor proporción de pacientes desnutridos en el grupo 1. Las herramientas de cribado nutricional MUST, NRI y MST eran más sensibles que los métodos objetivos. La medición del ángulo de fase fue el método objetivo más sensible en el grupo 1.
Conclusión: Las herramientas de cribado nutricional mostraron la mejor asociación con la EGS-GP y también fueron más sensibles que los métodos objetivos. Los resultados sugieren el uso de la combinación de MUST y EGS-GP en pacientes con cáncer antes y después del tratamiento.
Palabras clave: Evaluación nutricional. Evaluación Global Subjetiva Generada por el Paciente. Cáncer colorrectal. Cáncer gástrico. Pacientes ambulatorios.
Abbreviations
CRC: Colorectal cancer.
GC: Gastric cancer.
BMI: Body mass index.
MUST: Malnutrition Universal Screening Tool.
MST: Malnutrition Screening Tool.
NRI: Nutritional Risk Index.
PG-SGA: Patient-Generated Subjective Global Assessment.
SGA: Subjective Global Assessment.
PhA: Phase angle.
Introduction
Colorectal cancer (CRC) and gastric cancer (GC) are the most common cancers in the world.1,2 Gastrointestinal tract tumors can cause obstruction and impair nutrient absorption, events that result in weight loss.3 These patients therefore require adequate monitoring of nutritional status. Several methods are used for the assessment of nutritional status in cancer patients, but no gold standard exists since these methods are not specific for this group of patients and are influenced by factors that are independent of nutritional status.5 Within this context, nutritional status of cancer patients is evaluated using objective methods such as anthropometric and biochemical parameters, nutritional indices and body composition measures, nutritional screening tools, and subjective methods.6,7
The body mass index (BMI) is commonly used in epidemiological studies and clinical practice because of its simplicity, low cost, and satisfactory association with fat mass, morbidity and mortality, but shows low sensitivity in the diagnosis of undernutrition.8-10 Biochemical parameters such as serum albumin can also be used for nutritional assessment. However, changes in these parameters may occur due to the underlying disease and may not reliably reflect nutritional status.6
Phase angle is a parameter used in electrical bioimpedance analysis. A low phase angle suggests cell death or a decline in cell integrity. This parameter has been validated in several diseases, including cancer.11-13
The Malnutrition Universal Screening Tool (MUST) was developed for the detection of protein-calorie malnutrition and the identification of malnutrition risk using evidence-based standards.14 This instrument has been validated as a nutritional screening tool in cancer patients undergoing radiotherapy.15 The Malnutrition Screening Tool (MST) is a quick and simple nutritional screening tool based on weight loss and appetite changes, which has been validated for use in outpatients with cancer undergoing radiotherapy and inpatients.16,17 The Nutritional Risk Index (NRI) gained popularity because it differs from other assessment instruments by using objective parameters. This tool has been used for the evaluation of patients with different conditions and clinical outcomes and to monitor the impact of nutritional interventions. It was first used in hospitalized patients, but was later successfully applied to other groups of patients.18-20
The Patient-Generated Subjective Global Assessment (PG-SGA) tool21 is an adaptation of the Subjective Global Assessment (SGA),22 recommended by the Oncology Nutrition Dietetic Practice Group of the American Dietetic Association as standard for the nutritional assessment of cancer patients.23,24 It emphasizes symptoms commonly seen during the treatment of cancer and includes a physical examination for the subjective assessment of nutritional status.
In view of the highly prevalent nutritional depletion and importance of assessing nutritional status in cancer patients, the objective of the present study was to evaluate nutritional screening tools and subjective and objective methods for the identification of nutrition risk and nutritional status in patients with CRC and GC, and to compare the results to those obtained for patients treated for these cancers.
Patients and methods
Subjects
A cross-sectional study involving outpatients treated by the Oncology Group of the Gastroenterology Division, Federal University of São Paulo, was conducted between July 2010 and December 2011. Two different groups of patients were studied. The group 1 consisted of patients with CRC or GC with active disease undergoing or not chemotherapy and the group 2 consisted of patients under follow-up who had been treated for CRC or GC and who were tumor free for a period longer than 3 months.
The study was approved by the local Ethics Committee (Protocol 0826/10) and all patients signed an informed consent form.
Data collection
Data on gender, age, treatment and tumor were obtained from the medical records. The methods used in the study were applied on a single occasion, i.e., the patient was approached only once to assess nutritional variables and to collect blood for the determination of serum proteins.
Objective methods for the assessment of nutritional status
Weight (kg) and height (cm) were measured for the determination of BMI. The subjects were classified according to the World Health Organization criteria25 as undernourished (BMI < 18.5 kg/m2) or well nourished (BMI > 18.5 kg/m2).
The phase angle was calculated as the ratio between resistance (R) and reactance (Xc) determined with a Biodynamics 450® bioimpedance analyzer using a standard technique. R and Xc were measured directly in Ohms (Ω) at a single frequency of 50 kHz and 800 μA. The phase angle (PhA) was calculated using the following equation: PhA = arctan (Xc/R) x (180/3.14). The measurements were obtained early in the morning after a fast of at least 4 hours. All procedures and control for other variables affecting the validity, reproducibility and precision of the measurements were performed according to the National Institutes of Health guidelines.13
Serum albumin levels were measured by the bromocresol purple method (Biosystems®). The cut-off value was 3.5 mg/dL.26
Nutritional screening tools for the assessment of nutritional status
MUST uses three independent criteria for determination of the overall risk of undernutrition: BMI, percentage of weight loss over the previous 3-6 months, and if there has been or is likely to be no nutritional intake for > 5 days.14,15 The MST consists of two questions related to recent unintentional weight loss and low food intake because of decreased appetite. This tool provides a score between 0-5, with a score > 2 indicating a risk of undernutrition.16 The NRI was derived from serum albumin concentration and the ratio of actual to usual weight (1.519 x serum albumin, g/dL) + [41.7 x actual weight (kg)/ideal body weight (kg)]. Four categories of nutrition-related risk were defined: high risk, moderate risk, low risk, or no nutritional risk.18,19
Subjective method for the assessment of nutritional status
The validated Portuguese version of the scored PG-SGA was used to assess nutritional status.27 PG-SGA consists of two sections: (1) weight history, food intake, nutrition impact symptoms and functional capacity; (2) diagnosis, disease stage, age, components of metabolic demand (sepsis, neutropenic or tumour fever, corticosteroids) and physical examination. Subjective analysis classified the patients into three categories: (A) well-nourished, (B) moderately undernourished or suspected of being undernourished, and (C) severely undernourished. For the present study, and for between methods comparisons, two categories of the PG-SGA results were created: well nourished and undernourished if moderately or severely undernourished, to enable comparisons with other methods.15
The PG-SGA had been considered the gold standard to determine the sensitivity and specificity of the others methods used to evaluate the nutritional status. This tool had been validated for assessing nutritional status of patients with cancer21 and is the most complete and patient-related cancer instrument used in our study.
Statistical analysis
For descriptive statistics, quantitative variables are expressed as the mean and standard deviation and categorical (qualitative) variables as absolute and relative frequencies. The chi-squared test was used for comparison between groups and the Student t-test to compare continuous parametric variables. For the evaluation of phase angle, a cut-off value was established for the population studied because of the lack of specific values for cancer patients. The phase angle was divided by the distribution measured according to the proportion of observed frequencies. The data were separated into quartiles and values of the first quartile were defined as predictors of undernutrition.
Sensitivity and specificity of the methods used to assess nutritional status were calculated considering PG-SGA as the gold standard. The sensitivity test determines the proportion of true positives by the analysis of patients who are indeed undernourished, according to the PG-SGA. It is the proportion of individuals who have a positive result (undernourished,) when compared to the standard method of analysis and the total undernourished, by the PG-SGA. Specificity, in contrast, verified the ability of the methods to identify true negatives, analyzing the absence of undernutrition according to the standard method.
The test sensitivity was the proportion between the number of patients with BMI < 18.5 kg/m2, phase angle values in the first quartile, cut-off value for albumin < 3.5 mg/dL and "risk of undernutrition", by the screening tool MUST, MST and NRI and undernutrition diagnosed by PG-SGA. On the other hand the specificity refers to the proportion of patients without nutritional deficiency by the method studied compared to the well nourished by PG-SGA.
The chi-squared test was used to evaluate the degree of association between PG-SGA and the other methods. Statistical analysis was performed using the SPSS 16.0 program (2008, SPSS, Inc., Chicago, IL). A two-sided p-value < 0.05 was considered to indicate significance.
Results
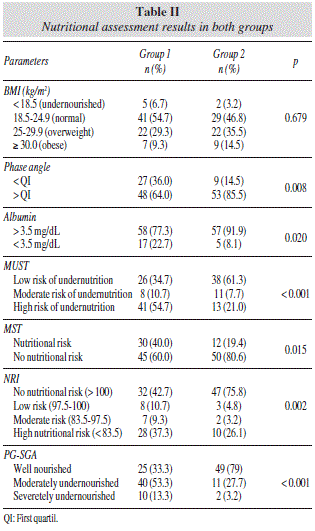
A total of 137 patients were eligible for the study (75 in group 1 and 62 in group 2) (table I). Advanced stage disease was more common on the group 1. In group 1, 40% of the patient had not received any treatment, 60% patients were receiving chemotherapy and of those 54.7% underwent surgery. Group 2 consisted of patients under follow up; 48.38% had received chemotherapy and all of them underwent surgical resection of the tumor. Comparing groups 1 and 2, the percentage of weight loss was 3.42 ± 4.86 versus 1.20 ± 2.30 in one month and 10.79 ± 9.73 versus 4.99 ± 8.88 in 6 months. The prevalence of moderate/severe undernutrition determined by the PG-SGA was 66.6% in group 1. According to BMI, only 6.7% of the patients were undernourished. Despite the nutritional assessment the BMI was the only method that revealed no significant difference (table II).
Significant associations (p < 0.05) were observed between the PG-SGA, considered a gold standard and most of the objective methods and nutritional screening tools used. The association between PG-SGA and the objective methods BMI in group 1 or albumin in group 2 were not significant (table III).
Analysis of the sensitivity and specificity of the methods used to assess nutritional status, calculated in relation to the PG-SGA showed low sensitivity, but high specificity, of the objective methods in the two groups. Phase angle measurement was the most sensitive method in group 1 (44%). MUST was the most sensitive nutritional screening tool (72% in group 1 and 84% in group 2) (table IV).
A high specificity of BMI (100% for both groups) had been observed because all the patients well nourished by this index had not undernutrition by the PG-SGA. Similarly most of the patients with normal serum levels of albumin were also well nourished by the PG-SGA (specificity of 92% for group 1 and 93.8% for group 2) (table IV).
Discussion
Patients with GC and CRC were included in this study because of the high prevalence of these cancers and of difficulties in the early identification of nutritional status since BMI can underestimate the current nutritional status of these patients.8 Colorectal cancer was more prevalent than GC in the two groups, in agreement with Global Cancer Statistics data.1 There were no patients with stage IV cancer in group 2 because the cure rate is low in advanced disease.1,2
All nutritional assessment methods tested revealed a significant difference between the two groups, except for BMI. This index had already been described as having lower sensitivity in the diagnosis of undernutrition.8-10
The risk of undernutrition were higher in patients with cancer (group 1) than in patients under follow-up (group 2) due to the disease stage and chemotherapy treatment. One consistent explanation for this finding is the fact that the presence of the tumor is more likely to cause nutrient depletion in the former.5
Among the 27 (26.3%) patients with a phase angle < first quartile (cut-off: 5.1), 22 (81.48%) were also classified as undernourished by the PG-SGA. In a study evaluating the relationship between phase angle and SGA in patients with advanced CRC, Gupta et al.28 obtained a cut-off similar to that of the present study (5.2), which showed 51.7% sensitivity and 79.5% specificity in detecting undernutrition. The authors concluded that PhA can be used as an indicator of nutritional status. In the present study, phase angle was found to be the most sensitive objective method for the diagnosis of undernutrition. It is therefore proposed that PhA can complement other nutritional data, predicting functionality, quality of life and prognosis in patients with cancer.29
Hypoalbuminemia occurs due to a systemic inflammatory response and nutrient depletion caused by the tumor.30 This condition is therefore more common among patients with metastases. In the present study, 62.7% of the patients had metastatic cancer and hypoalbuminemia was observed in only 34.0% of them. In contrast, albumin was identified as an indicator of nutritional risk in a study involving patients with metastatic cancer.6 In view of these divergent results, there is no consensus regarding the validity of serum albumin concentration as a parameter for the diagnosis of undernutrition.
In a recent validation of MUST involving 450 cancer patients under radiotherapy, including CRC patients,15 the percentage of patients at high nutritional risk (17%) was low. In the present study, MUST showed the highest sensitivity (72%) in the detection of nutritional risk in patients with cancer, but its specificity was low (48.9%). Boleo-Tome et al.15 also reported high sensitivity (80%) and specificity (89%) of this tool in relation to the PG-SGA. The percentage of weight loss, which is an important factor in the MUST questionnaire, may have been responsible for the differences between studies.15,31 The high prevalence of patients with increased nutritional risk found in the present investigation may be a consequence of the higher percentage of weight loss. Another factor that may have contributed in difference the studies were that Boleo-Tome et al15 evaluated predominantly patients with breast and prostate cancers, which are associated with lower weight loss compared to patients the present study.
Among the nutritional screening tools, the MST showed the lowest sensitivity and highest specificity. Higher sensitivity and specificity have been reported by Isering et al.32 who compared the MST and PG-SGA in oncology outpatients. This lower sensitivity observed in the present study might be due to the fact that the patients had a history of weight loss, but their current weight recovery was not computed in the standardized MST score.
Although the NRI is frequently used in hospital patients,18,19 in the present study this tool showed important sensitivity in the detection of undernutrition in outpatients. In a study on patients with GC who underwent curative gastrectomy, the NRI was considered a predictor of postoperative complications.33
A high prevalence of undernutrition was detected by the PG-SGA, with 66.6% of the patients in group 1 being classified as moderately or severely undernourished. Another study including CRC patients undergoing chemotherapy, with a similar percentage of stage IV cancer, reported a lower frequency of patients with moderate or severe undernutrition (42.4%).34 This difference might be due to the inclusion of patients with GC in the present study, who usually present a higher risk of undernutrition.35
We chose to determine the sensitivity and specificity of nutritional assessment methods in relation to the PG-SGA since the effectiveness of the latter has been demonstrated in several studies, specifically in cancer patients.21,24,31 The PG-SGA was able to identify patients at nutritional risk and can therefore be considered a nutritional screening method.27 Bauer et al.24 also reported a higher sensitivity and specificity of the PG-SGA compared to the standard SGA in hospitalized patients with cancer. Significant and similar associations between the PG-SGA and most nutritional screening variables were observed in the two groups. However, these associations were lower for the objective methods. The predominance of significant associations between the nutritional screening tools and PG-SGA may be due to the pre-established relationship between these methods. These results suggest the maintenance of nutritional risk assessment by nutritional screening tools, and if the presence of a nutritional risk is confirmed, the patient should undergo complete nutritional assessment using the PG-SGA.
This study has some limitations such as the small number of patients with GC. Furthermore, the lower sensitivity and specificity of nutritional assessment observed in this study when compared to other reports may be due to the fact that the subjects were outpatients and to the predominance of patients with CRC in good general health. However, this is the first study comparing nutritional assessment methods between patients with cancer and patients with a history of cancer under follow-up.
In conclusion, the nutritional screening tools tested showed higher sensitivity and lower specificity than the objective methods in the assessment of nutritional status when the PG-SGA was used as gold standard. We suggest the combination of the nutritional screening tool MUST and PG-SGA for the assessment of nutritional status. Although the percentage of patients at nutritional risk or with moderate/severe undernutrition is high among cancer patients, these alterations are also observed in the group of already treated patients, a fact highlighting the need for assessing nutritional status in both groups.
Acknowledgements
The authors thank the volunteers who participated in this study.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90. [ Links ]
2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893-917. [ Links ]
3. Khalid U, Spiro A, Baldwin C, Sharma B, McGough C, Norman AR, et al. Symptoms and weight loss in cancer patients with gastrointestinal and lung cancer at presentation. Support Care Cancer 2007; 15: 39-46. [ Links ]
4. Isenring EA, Capra S, Bauer J. Nutritional intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer 2004; 91(3): 447-52. [ Links ]
5. Valenzuela-Landaeta K, Rojas P, Basfi-fer K. Nutritional assessment for cancer patient. Nutr Hosp 2012; 27: 516-23. [ Links ]
6. Pereira NB, D'Alegria BS, Cohen C, Portari PEF, Medeiros FJ. Comparison of the nutritional diagnosis, obtained through different methods and indicators, in patients with cancer. Nutr Hosp 2009; 24: 51-55. [ Links ]
7. Marín Caro MM, Gómez Candela C, Castillo Rabaneda R, Lourenço Nogueira T, García Huerta M, Loria Kohen V, et al. Nutritional risk evaluation and establishment of nutritional support in oncology patients according to the protocol of the Spanish Nutrition and Cancer Group. Nutr Hosp 2008; 23: 458-68. [ Links ]
8. Li H, Yang G, Xiang YB, Gao J, Zhang X, Zheng W, et al. Body weight, fat distribution and colorectal cancer risk: a report from cohort studies of 134 255 Chinese men and women. Int J Obes (Lond) 2012 Sep 18. [ Links ]
9. Thibault R, Genton L, Pichard C. Body composition: Why, when and for who? Clin Nutr 2012; Jan 30. [ Links ]
10. Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One 2012; 7 (4): e33308. [ Links ]
11. Paiva SI, Borges LR, Halpern-Silveira D, Assunção MC, Barros AJ, Gonzalez MC. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support Care Cancer 2010; 19: 187-92. [ Links ]
12. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gomez J, et al. Bioelectrical impedance analysis- part I: review of principles and methods. Clin Nutr 2004; 23: 1226-43. [ Links ]
13. NIH Consensus statement. Bioelectrical impedance analysis in body composition measurement. National Institutes of Health Technology Assessment Conference Statement. Nutrition 1994; 12: 749-62. [ Links ]
14. Elia M. Screening for malnutrition: a multidisciplinary responsibility. Development and use of the 'Malnutrition Universal Screening Tool' (MUST) for adults. Malnutrition Advisory Group, a Standing Committee of BAPEN. Redditch: BAPEN 2003. [ Links ]
15. Boléo-Tomé C, Monteiro-Grillo I, Camilo M, Ravasco P. Validation of the Malnutrition Universal Screening Tool (MUST) in cancer. Br J Nutr 2011; 6: 1-6. [ Links ]
16. Ferguson ML, Bauer J, Gallagher B, Capra S, Christie DR, Mason BR. Validation of a malnutrition screening tool for patients receiving radiotherapy. Australas Radiol 1999; 43:325-27. [ Links ]
17. Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999; 15: 458-64. [ Links ]
18. Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med 1991; 325: 525-32. [ Links ]
19. Buzby GP, Knox LS, Crosby LO, Eisenberg JM, Haakenson CM, McNeal GE, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in undernourished surgical patients. Am J Clin Nutr 1988; 47: 366-81. [ Links ]
20. Caccialanza R, Klersy C, Cereda E, Cameletti B, Bonoldi A, Bonardi C, et al. Nutritional parameters associated with prolonged hospital stay among ambulatory adult patients. CMAJ 2010; 23; 182 (17): 1843-9. [ Links ]
21. Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996; 12: 15-9. [ Links ]
22. Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? J Parenter Enteral Nutr 1987; 11: 8-13. [ Links ]
23. McCallum PD, Polisena C (eds): Patient-generated subjective global assessment, The Clinical Guide to Oncology Nutrition. The American Dietetic Association 2000. 11-23. [ Links ]
24. Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002; 56: 779-85. [ Links ]
25. Organización Mundial de la Salud. El estado físico: uso e interpretación de la antropometría. Genebra: OMS; 1995, p. 452. [ Links ]
26. Brackeen GL, Dover JS, Long CL. Serum albumin. Differences in assay specificity. Nutr Clin Pract 1989; 4 (6): 203-5. [ Links ]
27. González MC, Borges LR, Silveira DH, Assunção MCF, Orlandi SP. Validação da versão em português da avaliação subjetiva global produzida pelo paciente. Rev Bras Nutr Clin 2010; 25: 102-08. [ Links ]
28. Gupta D, Lis CG, Dahlk SL, King J, Vashi PG, Grutsch JF, et al. The relationship between bioelectrical impedance phase angle and subjective global assessment in advanced colorectal cancer. Nutr J 2008; 7-19. [ Links ]
29. Norman K, Stobäus N, Zocher D, Bosy-Westphal A, Szramek A, Scheufele R, et al. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr 2010; 92: 612-619. [ Links ]
30. Al-Shaiba R, McMillan DC, Angerson WJ, Leen E, McArdle CS, Horgan P. The relationship between hypoalbuminaemia, tumour volume and the systemic inflammatory response in patients with colorectal liver metastases. Br J Cancer 2004; 9: 205-07. [ Links ]
31. Parekh N, Lin Y, Dipaola RS, Marcella S, Lu-Yao G. Obesity and prostate cancer detection: insights from three national surveys. Am J Med 2010; 123: 829-835. [ Links ]
32. Isenring E, Cross G, Daniels L, Kellett E, Koczwara B. Validity of the malnutrition screening tool as an effective predictor of nutritional risk in oncology outpatients receiving chemotherapy. Support Care in Cancer 2006; 14: 1152-56. [ Links ]
33. Cheong AO, Dae HK, Seung JO, Min GC, Jae HN, Tae SS, et al. Nutritional risk index as a predictor of postoperative wound complications after gastrectomy. World J Gastroenterol 2012; 18: 673-678. [ Links ]
34. Heredia M, Canales S, Sáez C, Testillano M. The nutritional status of patients with colorectal cancer undergoing chemotherapy. Farm Hosp 2008; 32: 35-37. [ Links ]
35. Rey-Ferro M, Castano R, Orozco O, Serna A, Moreno A. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition 1997; 13:878-81. [ Links ]
![]() Correspondence:
Correspondence:
Mariana Abe Vicente.
Nutritonist. Master of Science.
Universidade Federal de São Paulo.
São Paulo, Brazil.
E-mail: marianaav@hotmail.com
Recibido: 11-I-2013.
Aceptado: 28-I-2013.