Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 supl.2 Madrid 2013
Improvement of C peptide zero BMI 24-34 diabetic patients after tailored one anastomosis gastric bypass (BAGUA)
Mejoría de pacientes diabéticos péptido C cero IMC 24-34 tras bypass gástrico una anastomosis (BAGUA) tallado
M. Garcia-Caballero1, J. M. Martínez-Moreno1, J. A. Toval1, F. Miralles2, A. Mínguez1, D. Osorio1, J. M. Mata1 and A. Reyes-Ortiz1
1Dept. of Surgery University Málaga
2Dept. of Internal Medicine. Associate University Hospital Parque San Antonio. Málaga. Spain
ABSTRACT
Background: Although bariatric surgery proved to be a very effective method in the treatment of patients in whose pancreas still produce insulin (type 2 diabetes), the accompanied metabolic syndrome and their diabetes complications, there is no information on the effect of this type of surgery in BMI24-34 patients when pancreas do not produce insulin at all (type 1, LADA and long term evolution type 2 diabetes among others).
Patients and methods: We report preliminary data of a serie of 11 patients all with a C-peptide values below 0.0 ng/ml. They were followed for 6 to 60 months (mean 19 months) after surgery. We studied the changes in glycemic control, evolution of the metabolic syndrome and diabetes complications after one anastomosis gastric bypass (BAGUA).
Results: All values relative to glycemic control were improved HbA1c (from 8.9 ± 0.6 to 6.7 ± 0.2%), FPG (Fasting Plasma Glucose) [from 222.36 ± 16.87 to 94 ± 5 (mg/dl)] as well as the daily insulin requirement of rapid (from 40.6 ± 12.8 to 0 (U/d) and long-lasting insulin (from 41.27 ± 7.3 U/day to 15.2 ± 3.3 U/day). It resolved 100% of the metabolic syndrome diseases as well as severe hypoglycaemia episodes present before surgery and improved some serious complications from diabetes like retinopathy, nephropathy, neuropathy, peripheral vasculopathy and cardiopathy.
Conclusions: Tailored one anastomosis gastric bypass in BMI 24-34 C peptide zero diabetic patients eliminated the use of rapid insulin, reduced to only one injection per day long-lasting insulin and improved the glycemic control. After surgery disappear metabolic syndrome and severe hypoglycaemia episodes and improves significantly retinopathy, neuropathy, nephropathy, peripheral vasculopathy and cardiopathy.
Key words: T1DN. LADA. One anastomosis gastric bypass. C-peptide. Metabolic syndrome. Micro-and macro-vascular diabetes complications.
RESUMEN
Introducción: Aunque la cirugía bariátrica ha demostrado ser un método muy eficaz en el tratamiento de pacientes diabéticos cuyo páncreas aún es capaz de producir insulina (diabetes tipo 2), así como del síndrome metabólico y las complicaciones relacionadas con la diabetes, no hay información sobre el efecto de este tipo de cirugía en pacientes IMC 24-34 cuando el páncreas no produce insulina en absoluto (tipo 1, tipo LADA y diabetes tipo 2 de larga evolución, entre otros).
Métodos: Presentamos datos preliminares de una serie de 11 pacientes todos con valores de Péptido C < 0,0 ng/ml. El seguimiento postoperatorio varia de 6 y 60 meses (media 19 meses). Estudiamos los cambios en el control de la glucemia, evolución del síndrome metabólico y complicaciones relacionadas con la diabetes tras bypass de una anastomosis (BAGUA).
Resultados: Mejoraron todos los valores relativos al control glucémico HbA1c (de 8,9 ± 0,6 a 6,7 ± 0,2%), FPG (Glucosa Plasmática Ayunas) (de 222,36 ± 16,87 a 94 ± 5 (mg/dl)) así como el requerimiento diario de insulina, tanto de insulina rápida (de 40,6 ± 12,8 a 0 U/día) como de insulina retardada (41,27 ± 7,3 U/día a 15,2 ± 3,3 U/día). Se resolvieron el 100% de las comorbilidades estudiadas y se mejoraron algunas complicaciones graves derivadas de la diabetes como retinopatía o nefropatía.
Conclusiones: El bypass gástrico de una anastomosis adaptado a pacientes diabéticos IMC24-34 con péptido C cero elimina el uso de insulina de acción rápida, reduce a una sola inyección diaria la insulina retardada y mejora el control glucémico. Tras la cirugía desaparecen el síndrome metabólico y los episodios severos de hipoglucemia, y mejora significativamente la retinopatía, neuropatía, nefropatía, vasculopatía periférica y cardiopatía.
Palabras clave: DMT1. LADA. BAGUA. Péptido-C. Comorbilidades.
Introduction
Intensive glucose control did not succeed in showing mortality or cardiovascular benefits as demonstrated two recent meta-analysis,1,2 but doubles the occurrence of hypoglycemia severe enough to warrant intervention,3 does not improve quality of life1,2 and is associated with "dead-in-bed" syndrome4 and 3.4-fold increased risk of death.5
Morbidity and mortality in type 1 diabetic patients derive mainly from advanced microvascular, neuropathic, and macrovascular complications, with the major clinical impact beginning 15-20 years after the onset of diabetes.6-9 The problem is that normally type 1 diabetes appear in these patients during the first years of life.
Metabolic Surgery offers hope and the possibility of a treatment for patients suffering from DM who are always at risk of developing the diabetes life threatening complications. The treatment of those complications can be very difficult to endure for patients and a new treatment that would minimize this, is eagerly awaited by these patients. Therefore, surgery for them is not just a way of treating their illness it might prevent or ameliorate the present treatments and their side effects, or treatment that they have to endure with consequent impacts on their daily quality of life.
However, the possibility of curing DM with surgery is limited. There are doubts as to its action mechanism, perioperative risk, possible side effects and long-term effectiveness, among other limitations. Diabetes patients are permanently looking for new advances that can help them to improve their quality of life and prevent the development of diabetes complications. When bariatric surgery was discovered as a viable alternative able to get a complete reversion of diabetes, many patients (including those with diabetes mellitus type 1 and C-peptide < 0.0 ng/ml) consulted the possibility of using this option to improve their current situation and prevent the future.
At this moment, the experience accumulated in type 1 diabetes (C Peptide 0,0 ng/ml) refer to only a few cases and in obese patients operated by bariatric procedures to solve their obesity, although, the surgery has demonstrated to solve or improve diabetes control and its complications.10-12 The results of these few studies describe the remarked effect of surgery on insulin sensitivity, not only due to weight loss, but also on the first days after the operation. The same effect that has also been described by many authors in patients with type 2 DM in obese13,14 as also in non obese patients.15-17
On the other hand the effect of bariatric procedures, especially gastric bypass, on metabolic syndrome and the evolution of diabetic complications (retinopathy, neuropathy, cardiopathy and peripheral vascular disease) seems to be more related to an extra effects of gastric bypass (which pathophysilogical mechanisms are unknown so far) that to the direct effect on diabetes.
A final question of IMC24-34 C Peptide zero diabetic patients that ask to be operated by gastric bypass for improving their health after we explained them that so far there is no evidence for supporting the surgery in their case was: in case the gastric bypass do not do anything on my diabetes, what are the consequences for my health?. And the answer is that apart for the operatory risk (morbi-mortality near to 0-0,16%-for obese and easier surgery in normal weight patients), long term negative consequences are really minimal as has been proven over decades performing this type of surgery,18 while every day appear more evidence of the positive effects,19,20 independent of the BMI.21
All these arguments: low risk surgery, desperate health situation and long term expectations of the patients (especially those that started type 1 DM in childhood22 and the possibility of a very positive effect that could improve their every day quality of life and prevent future devastating diabetes complications, and the decisive support of the patients in spite of the lack of direct evidence, prompted us to initiate this study.
We hypothesise that tailored BAGUA is able to improve glycaemic control, metabolic syndrome, severe hypoglycaemia and other diabetes complications in patients with C-peptide < 0.0 ng/ml without direct relationship with the weight loss.
To attempt to demonstrate this hypothesis, we performed the following studies in patients undergo tailored BAGUA: 1. To study the changes in blood glucose levels and glycosylated haemoglobin after laparoscopic tailored BAGUA. 2. To evaluate the needs of antidiabetic treatment after laparoscopic tailored BAGUA. 3. To assess the changes in weight and body mass composition after tailored BAGUA. 4. To study the changes in diet, exercise and daily quality of life. 5. To evaluate the evolution of the metabolic syndrome as well as diabetes complications present before surgery.
Patients and methods
Patients
We report a preliminary experience in 11 diabetes mellitus patients with C-peptide levels < 0.0 ng/ml who underwent tailored BAGUA. Seven of the patients had a BMI 24-29 and 4 patients BMI 30-34. Sixty four percent were male and 36% female with an age ranging from 17 to 76 years. Five of them suffered from type 1 DM with demonstrated positive antibodies, four were LADA and two long term evolution type 2 DM. They were followed for a mean period of 19 months (ranged between 6 to 60 months) after surgery. A complete description of the characteristics of the patients population is summarized in tables I and II.
Variables of the study
All patients completed a structured interview to obtain the following data: sex, age, weight, height, medical history, drug use, and prevalent diseases. In the same way it was recorded their dietary habits and physical activity. Body composition was determined by bioimpedance (TANITA(R) is effected by placing feet of the patient over the electrodes. It transmits the patient an electric current type alternate, of 800 LA and at a frequency of 50 MHz. It is accepted that the body conducts electricity through the lean tissue and fat is not conductive. Mathematically it can be calculated the proportion and the amount of lean body mass and fat mass from weight and height and body impedance. The variation of the hydration status modifies the results by affect the conductivity, being an error factor.
Blood samples were extracted from peripheral vessels by vein puncture after fasting for 12 hours. From this sample is determined the concentrations of glucose by visible ultraviolet spectrophotometry and the glycosylated hemoglobin by high performance liquid chromatography (HPLC). The normal values of our laboratory are: Fasting Plasmatic Glucose from 65 to 105 mg/dl and glycosylated hemoglobin of 4.3 to 6.1% (23-43 mmol/mol).
C-Peptide (human proinsulin connecting peptide) is a polypeptide of3,600 Da and 31-aminoacid that is synthesized in pancreatic islets β-cells. It is an excision product of insulin biosynthesis and serving to link and stabilize the A- and B-chains of the insulin molecule, thus enabling correct folding and interchain disulfide bond formation. Proinsulin is divided enzymatically to insulin and C-peptide, which is stored in the pancreas and is secreted in equimolar amounts.That makes it a useful marker of insulin release because, unlike insulin, C-peptide is not extracted by the liver, but goes entirely to the bloodstream. Another C-peptide advantage is that its determination is not affected by insulin autoantibodies presence, which are frequently in patients treated with insulin. Beta-cell function, measured as C-peptide, is well recognised in autoimmune diabetes both through its correlation with endogenous insulin secretion and in relation to complications.22,23 Also in non-autoimmune diabetes, interest in Beta-cell function has recently risen considerably.24,25 The levels of C-peptide were determinate by immunological methods. Normal values are 0.8 to 4 ng/ml.
Furthermore we take 10 ml more samples for obtaining serum samples that are stored -80o C for future research purposes. These extractions are repeated 1 and 3 months for comparing the changes obtaining by diabetes surgery. We analyzed variables of lipid, cholesterol, HDL-cholesterol and triglycerides by visible ultraviolet spectrophotometry (LDL-cholesterol was obtained by the Friedewald formula). Normal levels in our laboratories are: Cholesterol from 130 to 220 mg/dl, HDL-cholesterol greater than 35 mg/dl, LDL-Cholesterol below 150 mg/dl and triglycerides between 45 and 185 mg/dl in men and between 40 and 160 mg/dl for women. Similarly, follow-up of the antidiabetic treatment and metabolic syndrome comorbidities, as well as the weight, body composition, eating habits, physical activity, DM complications (retinopathy, nephropathy, cardiopathy, neuropathy and peripheral vasculopathy).
Quality of life was determined by the validated spanish version Moorhead-Ardelt II questionnaire26 through successive postoperative interviews. The questionnaire have six questions scored from 1 to 10 points each. Good quality of life accounts from 42 to 60 points, medium 19-41 and bad 1-18.
Preoperative evaluation
All patients were subjected to a preoperative study following the indications of the Clinical Practice Guideline (CPG) of the European Association for endoscopic surgery (EAES).27 This study consists of an analytical of blood in which we studied the following parameters: complete blood count with differential leukocyte, blood type, glucose, urea, Na, Cl, K, Ca, clotting time and prothrombin activity, total cholesterol, HDL, triglycerides, alkaline phosphatase, AST, ALT, GGT and bilirubin, plasma cortisol, thyroid hormones: TSH, T3 and T4, total protein and proteinogramme, serum iron, B12 vitamin and antibodies anti-Helicobacter Pylori.
In addition there was a radiologic study, with abdominal ultrasound, Rx A-P and lateral chest and oesophagus-gastro-intestinal transit; cardiologic exploration with electrocardiogram (ECG) and stress and/or coronary ischemia tests (if applicable); functional respiratory tests and endoscope study (only in selected cases).
Before making the final decision we indicate the patients to contact with other type 1 DM patients already operated by BAGUA to treat their diabetes, for comment on self expectations and how was for the other the already operated patients. And so obtain information on what they could expect. Last question of the new patients to those already operated was if they will do surgery again, and unanimous answer was: yes.
Surgical procedure
All patients take only liquid diet during five days previous to surgery and received antibiotic and antithrombotic prophylaxis before surgery. The laparoscopic gastric bypass of single anastomosis (BAGUA)28 consists of the construction of a gastric pouch from the gastroesophageal junction to the end of the minor gastric curvature at the lower level of cisura angularis. The stapler line of the gastric pouch is fixed in approximately 12 cm to an intestinal loop (first layer of the antireflux mechanism) and anastomosed to it in a latero-lateral position excluding from the feeding course a length proportional to the BMI and distal to the Treitz ligament. Finally the antireflux mechanism is completed fixing the afferent loop to the gastric remnant and the efferent loop to the antrum. Both, the size of the gastric pouch and the length of bowel excluded depend on the BMI of the patient. In this group of patients the gastric pouch was always double as the size for obese patients and we excluded only 100 cm jejunum distal to the Treitz ligament for patients BMI 24-29, 120 cm BMI 30-32 and 150 cm BMI 33-34. We left systematically drainage during the 48 hours hospital stay.
Immediate postoperative care
First 24 hours patients received analgesics, antibiotics, low molecular weight heparin, procinetic, omeprazol and fluid-therapy. Patients are stimulated to start walking 8 hours after surgery. After the first 24 hours we retired all treatment except fluid-therapy and omeprazol. Around 48 hours after surgery we perform a gastrographic radiological test to check the gastro-intestinal anastomosis. If it is correct we start liquid diet and discharge patient home with only oral omeprazol and sucralphate. First week patient continues with liquid diet, second and third weeks every food pure and then start normal diet again.
Adjustment of the preoperative medical treatment
The diabetic treatment is adjusted according to the necessities that the patients had during the five days liquids diet that we indicated routinely as preparation for surgery. Patients with C Peptide < 0,0 ng/ml, starting with reduced dose of long-lasting insulin (1 to 10 iu) allowing during the first days a plasma glucose levels until 200 mg/dl and adjusting the dose progressively until as near as possible of normal values. This adjustment is done by phone contact as frequent as the patients need.
We indicated always the total abandon of antihypertension, anti-uricemic and antilypemic drugs, and exceptionally patients need taken treatment again and, if so, just some doses. Regarding anti-thrombotic medication when patient have stent implant or previous vascular accident, we reduced dose and/or drug classes according with the internist of the group (Dr. Miralles). We leave the control other diseases or diabetic complications, especially, retinopathy, nephropathy and cardiopathy to the correspondent specialities.
Follow up
The data were collected prospectively according with a previously fixed protocol. This protocol included a baseline evaluation preoperatively that studied parameters related to the evaluation of the disease, comorbidities, diabetic complications, weight and body composition. Similarly we took a sample of blood for the analysis of biochemical variables. After surgery by BAGUA (the procedure explained before) and the protocol outlined (diets, drugs) follow up was performed in biochemical variables. Mean follow-up study was 17 months. Routinely we continue seeing the patients at 1, 3, 6, 12, 18 and 24 months and then yearly. In these patients a phone contact is open 24 hours if necessary.
Statistical analysis
The qualitative variables will be described through frequencies and percentages. Quantitative variables were analyzed by Student's T-test in the case of the variables with normal distribution. In all analyzes shall be deemed to be statistically significant p values less than 0.05. Analyzes carried out with the statistical package SPSS (version 15.0 for Windows, SPSS, Chicago, IL) and Excel 2007.
Results
Changes in body weight and body composition
All results obtained in relation to weight, BMI, and body fat, were as expected after a bariatric surgery intervention. We measured in all cases a reduction in both weight and the amount of body fat mass (table III and fig. 1). We obtained the largest decreases in those patients who had a higher BMI (table III). However, despite the initial difference in the patients weight on the study, all stabilized around a mean BMI of 21.6 ± 2.5 kg/m2. Three patients were not happy with the weight loss during the first postoperative year (NA, AM, MJG):" they wanted some kilos more".
Fat mass values obtained by bioimpedance were reduced in all cases. These changes were statistically significant (P < 0.001). They decreased from a mean value of 31.0 ± 4.2% (before surgery) to 14.9 ± 1.3% (after surgery). In addition there is a positive correlation between the decrease in these values and those obtained for triglycerides with a bilateral significance of 0.012 and a correlation coefficient of 0.526.
Quality of Life assessment by Moorehead-Ardelt II Questionnaire (MA-II)
• All patients were in the range good (42 to 60 points) after evaluation by MAII at six months from surgery. Although patients had a mean preoperative score of 47, corresponding to a good quality of life (except two patients in medium range), after surgery it improve until 52 (table IV).
Diabetes Severity Markers by Diabetes Type and Evolution
• HbA1c: A general improvement was observed in all study groups undergoing BAGUA (fig. 2) regardless of diabetes type. The mean value of HbA1c in patients with T1DM decreased from 8.3 ± 0.6% to 6.7 ± 0.4%, in patients with LADA of 9.3 ± 1.5% to 6.5 ± 0.2 % and in patient with T2DM decreased from 9.4 ± 0.6 to 7.2 ± 0.7%. None statistically significant differences between groups were found (P 0.05).
Glycosylated hemoglobin values decreased in all studied cases (fig. 3) without relation to years of DM evolution. In general, the mean preoperative HbA1c was 8.9 ± 0.6% and decreased to 6.7 ± 0.2% for a mean follow-up period of 19 months. This decrease was statistically significant (P=0.003).
• FPG (Fasting plasmatic glucose): Glucose levels decreased in the 3 patients classes (fig. 4), showing a FPG values of 211.20 ± 12.7 (mg/dl) before BAGUA and 93 ± 5 (mg/dl) after in DMT1 patients. In LADA patients the decrease was higher, 247.75 ± 44.3 (mg/dl) before the operation and 100 ± 11 (mg/dl) after. Finally, in T2DM patients the decrease was from 199.5 ± 11.5 (mg/dl) to 84 ± 14.5 (mg/dl).
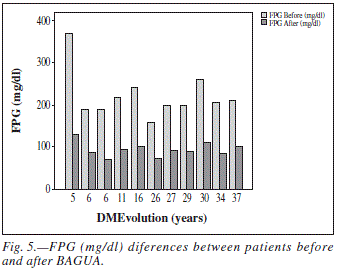
All patients in the study showed a decrease in FPG levels after surgery (fig. 5). Although this decrease was not related to the years of diabetes evolution. The values that were observed in the overall mean FPG levels before (222.36 ± 16.8 mg/dl) and after BAGUA (94 ± 5 mg/dl). This change is statistically significant (P = 0.00).
• Insulin: daily insulin patients requirement decreased in the 3 type of diabetes after the BAGUA (fig. 6). In T1DM patients the daily amount rapid insulin needed, decreased from 24.4 ± 2 to 0 and long lasting insulin requirement decreased from 36.2 ± 5 to 13.4 ± 3. In LADA patients, rapid insulin requirements were reduced from 66 ± 33.8 (U/Day) to 0 (U/Day). Daily long lasting insulin units required by these patients decreased from 56.5 ± 17.6 to 18.58 ± 8.7. Patients with T2DM also decreased their rapid insulin needs from 30.5 ± 9.5 to 0 (U/Day) and long lasting insulin from 23.5 ± 2.5 to 13 ± 3 (U/Day). No statistically significant differences were found between the three diabetes types.
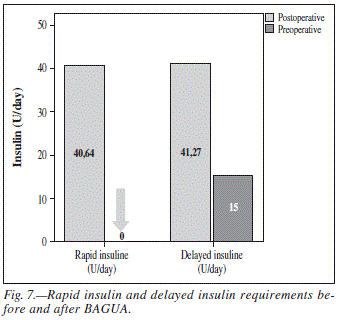
The required insulin units per day decreased in 11 patients after the BAGUA. This decrease was observed in both rapid insulin and long lasting insulin units (table V). Overall mean daily rapid insulin units needed before surgery was 40.6 ± 12.8 (U/day) and decreased to 0 (U/day) after surgery (fig. 9). This decrease was statistically significant (P = 0.01). Was also statistically significant (P = 0.00) the decrease in and long lasting insulin amount required by patients 41.3 ± 7.3 (U/day) at 15.3 ± 3.3 (U/day) (fig. 7).
Metabolic changes
We measured a general decrease in all the parameters of the lipid metabolism.
• Triglycerides: all study subjects had a decrease in triglyceride levels (fig. 8) and this decrease was even more pronounced in patients with hypertriglyceridemia (patients AR and AB). In these patients the decrease in triglyceride levels were 186 to 120 mg/dl in patient AR and from 198 to 97 mg/dl in the patient AB, both returning to normal values. Overall there was a decrease of 87.9 ± 17.21 mg/dl to 69.18 ± 8.1 mg/dl which was not statistically significant (P = 0.13). There is a positive correlation between the decrease in triglyceride levels and decreased body fat mass, with a two-sided significance of 0.012 and a correlation coefficient of 0.526.
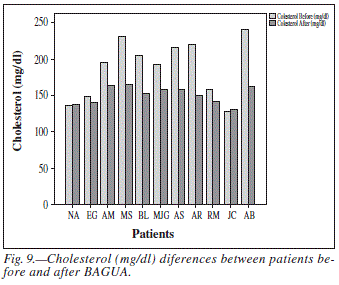
• Cholesterol, HDL-cholesterol y LDL-cholesterol: Total cholesterol values decreased from harmful to normal in patients with hypercholesterolemia (patient MS from 231 to 165 mg/dl and patient AB from 241 to 162 mg/dl) (fig. 9). In all other patients also a decrease was observed in total cholesterol levels but less marked. The general mean values decreased from 187 ± 12.16 to 150.81 ± 3.47 mg/dl (fig. 10). This decrease was statistically significant (P = 0.01). This decrease in total cholesterol correlated with LDL-cholesterol levels, with a Pearson correlation ratio of 0.9 and a two-sided significance of 0.00. Patient AB (only with LDL-cholesterol above normal) recovered normal values 161-100 mg/dl (fig. 10). Overall, there was a statistical significant change (P > 0.014) in the levels of LDL-cholesterol, which decreased from 108.72 ± 9.77 to 91.18 ± 4.51 mg/dl (fig. 10). The levels of HDL-cholesterol had not significant variations.
• Evolution of comorbidities: 8 of the patients presented one or more comorbidities before surgery (table VI). Dyslipidemia appeared in 2 patients; both of them used lipid-lowering drugs. Six patients were hypertensive and were treated by antihypertensive drugs. In 3 patients were detected harmful levels of cholesterol (HCO) requiring the use of medications. Uric acid high levels were observed in 3 patients and other 3 had an altered levels of GOT and GPT.
During the follow-up time, the resolution of comorbidities occurs in all patients undergoing BAGUA regardless of the evolution of DM.
• Complications: four patients in the study presented diabetes complications such as heart disease, retinopathy, nephropathy or peripheral vasculopathy (table VII). Retinopathy evolution was stopped according subsequent exams and nephropathy and vasculopathy were improved. Heart diseases also experiment an improvement reducing the necessary medication to a minimum. All patients suffered medium to severe hypoglycaemic crisis before BAGUA. After the BAGUA and during the monitoring time, these episodes disappeared 100% in all the patients.
Discussion
The treatment of type 1 diabetes is really challenging and many sophisticated alternatives are being suggested.30 The present conventional treatment implies very high costs31 and life threatening side effects.5
Conventional medical treatment try to avoid or delayed the development of diabetes micro- and macro-vascular complications that shorten the years of life of the patient. However the intensification of the treatment produce by itself new side effects that also increase the morbidity and mortality of the patients5,32 creating a difficult vicious circle.
But the treatment of diabetes have changed in the last years just by chance.33 Surgical changes in the gastrointestinal tract have demonstrated to be able to resolve or improve DM and the other metabolic disturbances present in many patients with only one therapeutic intervention.13-17 We do not know until now the exact mechanisms by which the effect is produced, but the good news for diabetic patients is that the effect is there.34 The effectiveness of surgery happen not only when the pancreas have still a normal function and the failure is due to an increased insulin resistance as is the case in simply of morbid obese patients, but also in patients insulin dependent in which the insulin production by the pancreas have already failed. The majority of the patients of our serie (putting together type 2 and those with C Peptide zero) were insulin dependent (67%)34 and in all of them tailored BAGUA had a positive effect on the glycemic control, coming to no necessity of treatment at all, or changing from insulin to oral antidiabetic drugs, or from great amount of insulin in several doses per day to only one injection per day of small amount.
This experience although small (only sixty five patients until now BMI 24-34 type 2 and type 1 DM) have produced very regular and repetitive results. Showing good correlations between the preoperative state of the pancreas (given by the values of fasten C Peptide) and the answer to surgery. This answer is not lineal and homogeneous. There is not a direct correlation between preoperative C Peptide levels and rapidity and intensity of the answer: resolution of DM without necessity of medication from surgery or transition period. And sometimes patients with lower C Peptide levels answered better than other with higher levels.
The years of disease have even worse correlation. There are patients with 20 years evolution and still in treatment with only oral anti-diabetic drugs, while other with only few years (less than ten) already need great amount of insulin and have developed severe micro- and macro-vascular complications.
The years of treatment with insulin translate, at least initially, time from the failure of pancreas for producing enough insulin. However, the evaluation of this data need to take into account the idea of the family doctor or endocrinologist responsible of the patient, to indicate a more or less intensive glycemic control. Or also the attention that the patient pay to his/her illness. Again we find great variability in the correlation of this parameter with the postoperative evolution of the patient.
In summary, from 60 first patients evaluated with a follow-up longer than 6 months,34 we find a 100% resolution (no treatment and HbA1c < 7%) in patients that only need oral anti-diabetic drugs preoperatively (n = 20,nine BMI 24-29 mean C Peptide 2,4 ng/ml and eleven BMI 30-34 mean C Peptide 3,5 ng/ml). From the 40 insulin dependent patients, the resolution rate was 50% (n = 20, five BMI 24-29 mean C Peptide 1,8 ng/ml and fifteen BMI 30-34 mean C Peptide 2,3 ng/ml). There were other 20 insulin dependent patients that only improve DM after surgery. Nine abandon insulin and needed only oral anti-diabetic drugs (n = 9, four BMI 24-29 mean C Peptide 1,02 ng/ml and five BMI 30-34 mean C Peptide 2,0 ng/ml). And 11 come from 3-4 injections of rapid and long lasting insulin per day to only one injection of long lasting insulin. Nine of these patients had C Peptide 0,0 ng/ml and are included in the sample analysed in this paper and other two patients had a C Peptide level of 0,88 and 1,17 ng/ml but continue needing one daily injection of long lasting insulin.
In our sample of 26 obese patients treated by BAGUA35 we found a similar postoperative evolution of the patients. Some of them needed oral anti-diabetics drugs to control glycemia and one obese patient (female) with type 1 diabetes reduced from four to one injection the insulin and improving the control of the nephropathy she suffered.
The lessons learned from this experience in type 2 DM and one obese patient with type 1 DM, and the 6 obese patients with type 1DM described in the literature,10-12 shows the same improvement after gastric bypass.
So, it seems to be three different situations in diabetes surgery: 1) Patients in treatment before operation with only oral anti-diabetics drugs that normally have variable period of DM evolution and an increased (depending of the degree of insulin resistance) or normal C Peptide level and presumably a healthy (still enough beta cell mass) but over stimulated pancreas that will cure DM after surgery; 2) Others patients already in treatment before operation with insulin, that normally have variable but longer period of DM evolution as well as variable period of insulin treatment and an increased (depending of the insulin resistance degree), normal or decreased C Peptide level and presumably damaged pancreas (limited beta cell mass) that can cure, need only oral anti-diabetic drugs or, rarely, one injection of minimal amount of long lasting insulin for controlling DM after surgery; and 3) Patients with no pancreas function at all, independent of the autoimmune or long lasting pancreas over load mechanism, also with variable period of DM evolution (although normally longer than in the previous described situations) and insulin treatment (that will depend of the genetic resistance of the different tissues and organs) that will need one injection of different amounts of long lasting insulin for controlling DM.
That means, from the point of view of the effect of diabetes surgery, type 1 diabetes have a different mechanism of damaging the pancreas function, that start earlier and that come sooner to total pancreas destruction. While LADA36 and type 2 diabetes provoke this destruction more slowly. But by both mechanisms the pancreas can come to a total destruction.
Thus could be explained why the effect of type 1 and type 2 DM on the body is similar,37 developing the same damage of the pancreas and organ complications31,32,37-39 and, hence, there is no logical reason to think that surgery will not have the same effect in type 1 as in type 2 diabetes based only in the different pancreas destruction mechanism.
The present paradigm in diabetes surgery is to operate only type 2 DM, and only those patients that could solve DM 100%. But, sometimes improvement is of central importance for the DM evolution and life expectancy of the patient. The Wisconsin Epidemiologic Study of Diabetic Retinopathy and a semi-Markov model predict a mortality of 51% at 10 years, prevalences of stroke and myocardial infarction of 18% and 19%, of nonproliferative diabetic retinopathy, proliferative retinopathy, and macular edema of 45, 16, and 18%, respectively. Microalbuminuria, proteinuria, and end-stage renal disease were predicted to be 19, 39, and 3%, respectively. Clinical neuropathy and amputation 52 and 5%, respectively, at 10 years. Over 10 years, average undiscounted total direct medical costs were estimated to be 53,000 US dolars per person.40 We think it is worth to examine the role of gastrointestinal surgery, which already have proved to ameliorate type 2 DM, for improving this disaster and costly evolution of patients with C Peptide zero, that means no pancreas function at all.
This simple and logical a priori appreciation, that type 1 diabetes will have a positive answer to gastric bypass, has been confirmed by the results of the present study. There was a positive effect on glycemic control and metabolic syndrome resolution without major complications and no mortality, similar to that obtained previously in type 2 DM operated by BAGUA.17,34 There was not excessive weight loss or long term digestive side effects as was also observed in type 2 DM BMI 24-34 patients.17,34,41 And the quality of life of the patients improved.
It is very interesting from the point of view of the mechanisms by which the gastrointestinal changes induced by the gastric bypass act on diabetes resolution even without any internal insulin production (as happen in all these patients).
However, the heterogeneous evolution of DM described above could be understand if we look into the complex mechanisms of glucose metabolism very nice explained in other papers of this issue. A fail in one or more of the many steps of this complex process, could conditioned different degrees and intensity of malfunction. After gastric bypass surgery it seems to be two different pathways for controlling glucose metabolism: one of them is pancreas depending; and the other is pancreas independent and is related to the derivation of food to the distal intestine and the consequent release of glucose into the portal blood. Which induce a brain response that enhanced the suppression of hepatic glucose production by insulin.42 These changes demonstrated in animals and humans43-45 could also explained the postive effect of surgery in absence of pancreas function.
So, could be explained that these patients do not need rapid insulin after surgery. And that they could control the glycaemia levels with only one injection per day of 4 to 10 fold less long lasting insulin.
But other questions are open as: what is the mechanism by which improve the evolution of the clinically established complications as cardiopathy, retinopathy, nephropathy, peripheral vasculopathy, neuropathy and sexual impotence after BAGUA? Is only a consequence of the better diabetes control, reduced use of insulin, or are specific gastric bypass effects? What is the role of the degree of organ damage in the improvement of clinically established diabetes complications in the postsurgical amelioration and what are the mechanisms by which this amelioration developed?
In summary, what these results pointed out is that the gastrointestinal tract play a central role in the regulation of glucose metabolism (as is also reported in other papers of this monographic issue) and that this effect is independent of pancreas function.
The present results, that should be confirmed by other similar experiences, could suppose an important help in the difficult management of type 1 and other type of diabetes in which pancreas has been destroyed.
Conclusions
One anastomosis gastric bypass (BAGUA) appears to be a real alternative for treating patients without any pancreas function (C-peptide < 0.0 ng/ml). Improving glycemic control, resolving the metabolic syndrome, and improving the serious complications of the disease such as cardiopathy, retinopathy, nephropathy, peripheral vasculopathy, neuropathy and sexual impotence. However, further studies are needed with larger series and longer follow-up periods in order to make a real assessment of the effect of this type of surgery on these patients.
References
1. Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; (6): CD008143. [ Links ]
2. Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassa'i B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 2011; 26: 343-345. [ Links ]
3. Realsen JM, Chase HP. Recent advances in the prevention of hypoglycemia in type 1 diabetes. Diabetes Technol Ther 2011; 13: 1177-86. [ Links ]
4. Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the "dead-in-bed" syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract 2010; 16: 244-8. [ Links ]
5. McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012; 35: 1897-901. [ Links ]
6. Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 1983; 25: 496-501. [ Links ]
7. Jacobs J, Sena M, Fox N. The cost of hospitalization for the late complications of diabetes in the United States. Diabet Med 1991; 8: S23-S29. [ Links ]
8. García-Caballero M, Valle M, Martínez-Moreno JM, Miralles F, Toval JA, Mata JM, Osorio D, Mínguez A. Resolution of diabetes mellitus and metabolic syndrome in normal weight 24-29 BMI patients with one anastomosis gastric bypass. Nutr Hosp 2012; 27 (2): 633-64. [ Links ]
9. Reyes García R, Romero Muñoz M, Galbis Verdú H. Bariatric surgery in type 1 diabetes (spanish). Endocrinol Nutr 2013; 60 (1): 46-47. [ Links ]
10. Mendez C.E., Tanenberg R. J. and Pories W. Outcomes of Roux-en-Y gastric bypass surgery for severely obese patients with type 1 diabetes: a case series report. Diabetes, Metabolic 28. Syndrome and Obesity. Targets and Therapy 2010; 3: 281-283. [ Links ]
11. Czupryniak L, Strzelczyk J, Cypryk K, Pawlowski, Maciej;et al. Gastric Bypass Surgery in Severely Obese Type 1 Diabetic Patients. Diabetes Care 2004; 27: 2561-4. [ Links ]
12. Czupryniak L, Wiszniewski M, Szymanski D, et al. Long-term results of gastric bypass surgery in morbidly obeses type 1 diabetes patients. Obes Surg 2010; 20: 506-508. [ Links ]
13. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J 30. Med 2009; 122: 248-256. [ Links ]
14. Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, 31. Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366: 1567-76. [ Links ]
15. Fried M, Ribaric G, Buchwald JN, Svacina S, Dolezalova K, Scopinaro N. Metabolic surgery for the treatment of type 2. diabetes in patients with BMI < 35 kg/m2: An integrated review of early studies. Obes Surg 2010; 20: 776-790. [ Links ]
16. Reis CE, Alvarez-Leite J, Bressan J, Alfenas RC. Role of Bariatric-Metabolic Surgery in the Treatment of Obese Type 2 Diabetes with Body Mass Index < 35. A Literature Review. Diabetes Technol Ther 2012; 14: 1-8. [ Links ]
17. García-Caballero M, Valle M, Martínez Moreno J, Miralles F, 34. Toval JA, Mata JM, Osorio D, Minguez A. Resolution of diabetes mellitus and metabolic syndrome in normal weight 24-29 BMI patients with one anastomosis gastric bypass. Nutr Hosp 2012; 27: 623-631. [ Links ]
18. Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery 2007; 142: 621-32. [ Links ]
19. Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg 2011; 253: 484-7. [ Links ]
20. Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson B, Jacobson P, Lönroth H, Maglio C, Näslund I, Pirazzi C, Romeo S, Sjöholm K, Sjöström E, Wedel H, Svensson PA, Sjöström L. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012; 367: 695-704. [ Links ]
21. Sjöholm K, Anveden A, Peltonen M, Jacobson P, Romeo S, Svensson PA, Sjöström L, Carlsson LM. Evaluation of Current Eligibility Criteria for Bariatric Surgery: Diabetes prevention and risk factor changes in the Swedish Obese Subjects (SOS) study. Diabetes Care 2013. (Epub ahead of print) [ Links ]
22. McVeigh GE, Gibson W, Hamilton PK. Cardiovascular risk in the young type 1 diabetes population with a low 10-year, but high lifetime risk of cardiovascular disease. Diabetes Obes Metab 2013; 15: 198-203. [ Links ]
23. Faber OK, Binder C. B-cell function and blood glucose control in insulin dependent diabetics within the first month of insulin treatment. Diabetologia 1977; 13: 263-268. (doi:10.1007/BF 01219710). [ Links ]
24. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003; 26832-836. (doi:10.2337/diacare.26.3.832). [ Links ]
25. Palmer AJ, Roze S, Valentine WJ, Minshall ME, Lammert M, Oglesby A, Hayes C, Spinas GA. What impact would pancreatic beta-cell preservation have on life expectancy, quality-adjusted life expectancy and costs of complications in patients with type 2 diabetes? A projection using the CORE Diabetes Model. Current Medical Research and Opinion 2004: 20 (Suppl. 1): S59-S66. [ Links ]
26. Marchetti P, Lupi R, Del Guerra S, Bugliani M, D'Aleo V, Occhipinti M, Boggi U, Marselli L, Masini M. Goals of treatment for type 2 diabetes: beta-cell preservation for glycemic control. Diabetes Care 2009; 32 (Suppl. 2): S178-S183. (doi:10.2337/dc09-S306). [ Links ]
27. Sauerland S, Weiner S, Hausler E, Dolezalova K, Angrisani L, Noguera CM, Garcíacaballero M, Immenroth M. Validity of the Czech, German, Italian, and Spanish version of the Moore-head-Ardelt II questionnaire in patients with morbid obesity. Obes Facts 2009; 2 (Suppl. 1): 57-62. [ Links ]
28. Sauerland S, Angrisani L, Belachew M, Chevallier JM, Favretti F, Finer N, Fingerhut A, Garcia-Caballero M, Guisado Macias A, Mittermair R, Morino M, Msika S, Rubino F, Tacchino R, Weiner R, Neugebauer EA; European Association for Endoscopic Surgery. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc 2005; 19: 200-21. [ Links ]
29. García-Caballero M, Carbajo M. One anastomosis gastric bypass: a simple, safe and efficient surgical procedure for treating morbid obesity. Nutr Hosp 2004; 19: 372-5. [ Links ]
30. Tudurí E, Bruin JE, Kieffer TJ. Restoring insulin production for type 1 diabetes. J Diabetes 2012; 4: 319-31. [ Links ]
31. Franciosi M, Lucisano G, Amoretti R, Capani F, Bruttomesso D, Di Bartolo P, Girelli A, Leonetti F, Morviducci L, Vitacolonna E, Nicolucci A. Costs of treatment and complications of adult type 1 diabetes. Nutr Metab Cardiovasc Dis 2012; 29. (Epub ahead of print). [ Links ]
32. Allemann S, Saner C, Zwahlen M, Christ ER, Diem P, Stettler C. Long-term cardiovascular and non-cardiovascular mortality in women and men with type 1 and type 2 diabetes mellitus: a 30-year follow-up in Switzerland. Swiss Med Wkly 2009; 139: 576-83. [ Links ]
33. Garcíacaballero M. Type 2 diabetes surgery: A casual finding? Cir Esp 2010: 88: 355-7. [ Links ]
34. García-Caballero M, Martínez-Moreno JM, Toval JA, Miralles F, Mata JM, Osorio D, Mínguez A. Diabetes Mellitus with Metabolic Syndrome in BMI 24-29 vs 30-34 treated by One Anastomosis Gastric Bypass: is there differences in the results? XVII World Congress IFSO2012. 11-15 Sept New Delhi. Final Programme Book page 44. [ Links ]
35. García-Caballero M. Results of one anastomosis gastric bypass as treatment of diabetes mellitus type 2 in obese: its relation with surgical difficulty and prerioperative complitations. In: Diabetes Surgery. Garciacaballero M, Tinahones FJ, Cohen R (ed). McGraw-Hill. Madrid 2010. Pages:147-161. [ Links ]
36. Brophy S, Davies H, Mannan S, Brunt H, Williams R. Interventions for latent autoimmune diabetes (LADA) in adults. Cochrane Database Syst Rev 2011; (9): CD006165. [ Links ]
37. Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care 2008; 31: 714-9. [ Links ]
38. Enzlin P, Rosen R, Wiegel M, Brown J, Wessells H, Gatcomb P, Rutledge B, Chan KL, Cleary PA; DCCT/EDIC Research Group. Sexual dysfunction in women with type 1 diabetes: long-term findings from the DCCT/EDIC study cohort. Diabetes Care 2009; 32 (5): 780-5. [ Links ]
39. Grauslund J. Eye complications and markers of morbidity and mortality in long-term type 1 diabetes. Acta Ophthalmol 2011; 89 Thesis 1: 1-19. [ Links ]
40. Zhou H, Isaman DJ, Messinger S, Brown MB, Klein R, Brandle M, Herman WH. A computer simulation model of diabetes progression, quality of life, and cost. Diabetes Care 2005; 28 (12): 2856-63. [ Links ]
41. Scopinaro N, Adami GF, Papadia FS, Camerini G, Carlini F, Fried M, Briatore L, D'Alessandro G, Andraghetti G, Cordera R. Effects of biliopanceratic diversion on type 2 diabetes in patients with BMI 25 to 35. Ann Surg 2011; 253: 699-703. [ Links ]
42. Troy S, M Soty, Ribeiro L et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not gastric after lap-band in mice. Cell Metab 2008, 8: 201-11. [ Links ]
43. Delaere F, Magnan C, Mithieux G. Hypothalamic integration of portal glucose signals and control of food intake and insulin sensitivity. Diabetes & Metabolism 2010: 36: 257-62. [ Links ]
44. Hayes MT, Foo J, Besic V et al. Is intestinal gluconeogenesis a key factor in the early changes in glucose homeosatasis following gastric bypass? Obes Surg 2011; 21: 759-62. [ Links ]
45. Svelikova E, Zahiragic S, Pieber TE et al. Improved glucose metabolism early after gastric bypass surgery relies primarily on enhanced insulin sensitivity. Diabetologia 2011; 54 (Suppl. 1): S83. [ Links ]
![]() Correspondence:
Correspondence:
M. García-Caballero
Department of Surgery
Facultad de Medicina
29080 Málaga. Spain
E-mail: gcaballe@uma.es