Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.29 no.5 Madrid may. 2014
https://dx.doi.org/10.3305/nh.2014.29.5.7290
ORIGINAL / Síndrome metabólico
Metabolic disorders of liver and iron in diabetic and non-diabetic patients BMI < 35 or > 35 before gastric bypass
Trastornos del metabolismo del hígado y el hierro en pacientes diabéticos y no diabéticos con IMC < 35 ó > 35 antes de bypass gástrico
Manuel Garciacaballero1, Alexander Reyes-Ortiz1,2,3, José Manuel Martínez-Moreno1 and José Antonio Toval-Mata1
1 Dept. of Surgery, Medical Faculty. Malaga. Spain.
2 Autonomous University of the State of Mexico.
3 U.M.F. 229, Del. 16. Mexican Social Security Institute. Mexico.
ABSTRACT
Introduction: The presence of abnormalities in the metabolic pathways of iron and liver functioning can produce insulin resistance or metabolic syndrome. Therefore, it is important to examine those alterations that may lead to the development of diseases. Nutritional status is another important factor that is intimately linked to diabetes and obesity.
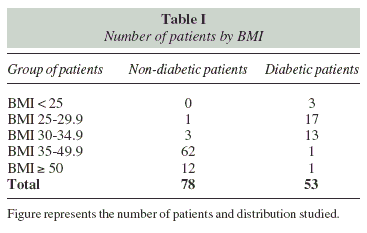
Patients and Methods: We studied 131 patients (78 nondiabetic patients and 53 diabetic), 37 patients BMI ≤ 35 (3 BMI < 25, 18 BMI 25-29.9, 16 BMI 30-34.9) and 94 patients BMI ≥ 35 (81 BMI 35-49.9 and 13 BMI ≥ 50). Subjects underwent to laboratory studies related to liver functioning and iron metabolism. Nutritional status was also determined in our patients.
Results: Iron was altered 14% of patients BMI >35 and diabetics reached 3% to 25%. Vitamin B12 was low 4% of non-diabetics BMI > 35, and high in 6% of diabetics BMI < 35. The 6% of diabetics BMI < 35 had hyperbilirubinemia. Transaminases are elevated in patients BMI >35 but exacerbated on diabetics. GGT is raised 41% to 47% in BMI >35. ALP is elevated in 25% of diabetics. Total protein and serum albumin were altered in diabetics causing mild malnutrition. 90% of patients had normal nutrition and 10% mild malnutrition.
Conclusions: The metabolisms Hepatic and iron are closely related to the onset of obesity and diabetes. If there is weight gain, cumulative metabolic risks rise. The presence of diabesity and increased duration of diabetes produce altered metabolism. Nutritional status is altered in obesity but is worse with the addition of diabetes.
Key words: Obesity. Diabetes mellitus. Diabesity. BMI. Metabolism.
RESUMEN
Introducción: La presencia de anormalidades en las vías metabólicas del hierro y el funcionamiento del hígado pueden producir resistencia a la insulina o síndrome metabólico. Por lo tanto, es importante examinar esas alteraciones que pueden conducir al desarrollo de enfermedades. El estado nutricional es otro factor importante que está íntimamente ligada a la diabetes y la obesidad.
Pacientes y métodos: Se estudiaron 131 pacientes (78 pacientes no diabéticos y 53 diabéticos), 37 pacientes IMC ≤ 35 (3 IMC < 25, 18 IMC 25-29,9, 16 IMC 30-34,9) y 94 pacientes IMC ≥ 35 (81 IMC 35-49,9 y 13 de IMC ≥ 50). Los sujetos fueron sometidos a estudios de laboratorio relacionadas con el funcionamiento del hígado y el metabolismo del hierro. Se determinó también el estado nutricional en nuestros pacientes.
Resultados: El hierro estuvo alterado en 14% de los pacientes IMC >35 y los diabéticos alcanzaron 3% a 25%. La vitamina B12 fue baja en 4% de los no diabéticos IMC > 35, y alta en el 6% de los diabéticos IMC < 35. El 6% de los diabéticos IMC < 35 tenía hiperbilirrubinemia. Las transaminasas estuvieron elevadas en pacientes IMC > 35, pero exacerbados en los diabéticos. GGT se eleva del 41% al 47% en BMI > 35. ALP estuvo elevada en el 25% de los diabéticos. Las proteínas séricas totales y la albúmina estuvieron alterados en los diabéticos causando desnutrición leve. 90% de los pacientes tenían una nutrición normal y 10% desnutrición leve.
Conclusiones: El metabolismo hepático y del hierro están estrechamente relacionadas con el inicio de la obesidad y la diabetes. Si hay un aumento de peso, los riesgos metabólicos acumulados se elevan. La presencia de la diabesidad y el aumento de la duración de la diabetes empeoran el metabolismo. El estado nutricional se altera en la obesidad, pero es peor con la adición de la diabetes.
Palabras clave: Obesidad. Diabetes mellitus. Diabesidad. IMC. Metabolismo.
Introduction
Alterations of metabolic pathways in the human body can cause several diseases, so it is necessary to explore the relationship between the predisposition, onset, progression and the etiology among alterations of metabolism that can lead to develop diseases1. Behind obesity and Type 2 Diabetes Mellitus (T2DM), a complex metabolic disorder causes a strong link between the two diseases2 on an intricate environment3. The increase in body weight predominantly visceral fat accumulation causes alterations as ectopic triglycerides4 which can cause insulin resistance and metabolic syndrome (MS), factors that favor the onset of T2DM4,5 in genetically predisposed individuals with impaired beta cell through the long process of the defects in insulin action and secretion6.
Obesity therefore is an important factor for the development of T2DM considered epidemic by the very high rates worldwide7. Today, half of patients with T2DM diagnosis are obese2, showing altered metabolic states and oxidative stress with inflammatory cytokines released by adipose tissue8.
Other metabolic pathways linked to the development of diabetes are the iron and hepatic metabolism altered. The increased total levels of stored iron are positively correlated with insulin resistance and metabolic syndrome8. The association of impaired liver enzymes with development of diseases has been demonstrated. Raising the glutamyltransferase (GGT) and alanine aminotransferase (ALT) are linked to peripheral and hepatic insulin resistance9. Elevation of bilirubin is considered as antioxidant in the prevention of cardiovascular diseases10. Higher levels of alkaline phosphatase (ALP) circulating is closely linked to body fat so it can be used as a predictor of obesity11.
Besides, deficiency of vitamin B12 is linked to the use of metformin in obese and/or patients with T2DM causes diverse clinical manifestations12.
Both obesity and/or diabetes can cause malnutrition13, and have a direct impact on the clinical outcome of patients. Diagnosis of obesity is made by Body Mass Index (BMI)14 which is considered as the main tool for the classification of weight but it is not related to the nutritional status of patients15. Ulibarri et al16 shows that 30 to 55% of hospitalized patients and 77 to 84% of diabetic patients have malnutrition, which increases morbidity and worsens the prognosis of patients by taking them to complications or death13.
Therefore, obesity should be prevented from an early age, because of morbidity increases with the time of evolution, and reverse it is a real challenge17 that could be achieved through bariatric surgery that are considered as definitive treatment of obesity and T2DM2. Metabolic surgery causes high rates of remission of T2DM and diseases such as MS18 by different mechanisms. The reorganization of gastrointestinal transit19 and visceral fat reduction are the goals of treating obesity4 and diabetes19. Schauer et al20 evaluated the specific effects of bariatric surgery on BMI > 35, and others authors on BMI < 3521,22. However, bariatric surgery treatment is still controversial on BMI < 3523 despite the results of long-term remission of obesity and diabetes as well as protective against T2DM24.
Patients and methods
Patients
We studied 131 patients (78 non-diabetic patients and 53 diabetic), 37 patients BMI ≤ 35 (3 BMI < 25, 18 BMI 25-29,9, 16 BMI 30-34,9) and 94 patients BMI ≥ 35 (81 BMI 35-49,9 and 13 BMI ≥ 50). We analyzed BMI, gender, presence of diabetes and its time of evolution. Age was clustered in ranges 15-20, 21-30, 3140, 41-50, and 51 years of age and older. The duration of T2DM was clustered into 1-5, 6-10, 11-15, 16-20, 21-30 and 31-40 years.
Patients attended medical consultation in order to be undergoing to tailored One Anastomosis Gastric Bypass (BAGUA) for his current state of diabetes, obesity or both conditions.
Preoperative evaluation
In all patients, a preoperative study were conducted following the indications of the Clinical Practice Guideline (CPG) of the European Association for Endoscopic Surgery (EAES)25.
The total cholesterol, serum iron, vitamin B12, total bilirubin, transaminase AST, transaminase ALT, gamma glutamyltransferase (GGT), alkaline phosphatase (ALP), total protein, serum albumin and total lymphocyte were evaluated after at least 12-hour fasting.
Surgical procedure
Before surgery, all patients eat only liquid diet during 5 (BMI 23-34) to 7 days (BMI 35-50) depending of preoperative BMI, received antibiotic and antithrombotic prophylaxis. Tailored BAGUA19,26 consisted in the construction of a gastric pouch from the gastroesophageal junction to the end of the minor gastrie curvature at the lower level of the cisura angularis. The stapler line of the gastric pouch was fixed approximately 12 cm to the intestinal loop (first layer of the anti-reflux mechanism of BAGUA), and it was anastomosed in a laterolateral position to the mesenteric border of a small bowel loop 100 cm (BMI 23-29), 120 cm (BMI 30-32), 150 cm (BMI 33-34), 200 cm (BMI 35-40), 250 cm (BMI 40-45), and 280 cm distal (BMI 45-50) to the treitz ligament. The anti-reflux mechanism is completed fixing the afferent loop to the gastric remnant and the efferent loop to the antrum.
The size of the gastric pouch depends on the BMI of the patient. In BMI 23-32 we performed a floppy 36 French bougie pouch, while in BMI > 33 a narrow 36 French bougie one's is performed. We left systematically a drainage during the 48 h of hospital stay. After surgery, they eat liquid diet in the 1st week, semifluids in 2nd week, puree in 3-4th weeks and normal diet after one month of surgery. The patient were reviewed at 10 days, 1, 3, 6 and 12 months.
Blood sample processing
Laboratory personnel of the Analysis Unit Clinical Laboratory of University Associated Hospital Parque San Antonio performed the extraction of blood samples from patients with at least 12 hours fasting. The laboratory samples analyzed were total cholesterol, total bilirubin, transaminases AST and ALT, GGT, ALP, serum iron and total protein by ultraviolet-visible spectroscopy. The serum albumin was analyzed by capil lary electrophoresis, vitamin B12 by chemiluminescence, and total lymphocyte by count and electronic formula.
Normal levels of our laboratory are total cholesterol 130-220 mg/Dl, total bilirubin < 1,2 mg/Dl, GGT 7-35 U/L, AST < 40 U/L, ALT < 40 U / L, ALP 30-120 U/L, total protein 65-82 g/L, serum albumin 36-54 g/L (3,65,4 g/Dl), serum iron 40-150 μg/Dl, vitamin B12 189883 pg/Ml and total lymphocytes 900-5175/μL.
With the data obtained we made the determination of the nutritional status of patients through screening tool developed by Ulibarri et al16 validated in 2002 with 92,3 sensitivity and specificity of 85.
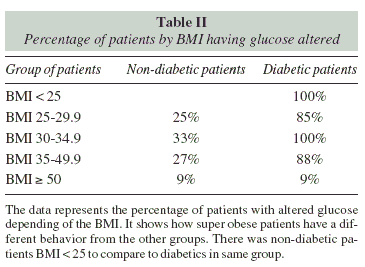
The filter tool uses serum albumin and total cholesterol as biochemical parameters, and uses total lymphocytes as immune parameters. The levels of the three parameters are set according to the degree of malnutrition. Assigned scores are divided into four ranges based on alteration of each laboratory studies (table II).
Statistical analysis
Descriptive statistics was used for comparisons. Differences among groups were analyzed by ANOVA when appropriate, and measures of central tendency according to study variables. Besides, we use percentages on gender, age, BMI, presence or absence of diabetes and its evolution time. For quantitative variables were used mean and standard deviation. We use SPSS (version 20 for Windows, SPSS, Chicago IL) and Excel 2010 programs.
Results
The predominant age range was 51 years and older (32% of patients). The mean age was 44 ± 13 years. The mean of BMI was 39 ± 14. The mean of T2DM evolution was 13 ± 8 years. Morbid obesity was predominant (61% of patients).
Non-diabetic patients had total cholesterol 208 ± 45 mg/dl, serum iron 78 ± 32 μg/dL, vitamin B12 414 ± 190 pg/mL, total bilirubin 2 ± 14 mg/dl, AST 25 ± 13 U/L, ALT 37 ± 27 U/L, GGT 42 ± 36 U/L, ALP 89 ± 25 U/L, total proteins 73 ± 3 g/L, serum albumin 4 ± 0 g/L, and total lymphocytes 2,546 ± 773 L.
Diabetic patients had total cholesterol 188 ± 40 mg/dl, serum iron 77 ± 32 μg/dL, vitamin B12 489 ± 419 pg/mL, total bilirubin 3 ± 11 mg/dl, AST 27 ± 15 U/L, ALT 38 ± 29 U/L, GGT 51 ± 50 U/L, ALP 84 ± 29 U/L, total protein 74 ± 4 g/L, serum albumin 4 ± 0 g/L and total lymphocyte 2,504 ± 737 μL.
The iron metabolism was altered in 20% of non-diabetic BMI < 35, but the 13% of patients with BMI > 35 was affected. Diabetic patients reached levels of 7% in BMI < 35, but increases to 26% with gain of weight (BMI > 35). Vitamin B12 is altered in certain groups of patients as you can see in table III.
We found hyperbilirubinemia in 6% of diabetic patients BMI < 35. The transaminases AST and ALT are elevated in high percentage of patients BMI > 35 but exacerbated on diabetics. GGT is raised from 41% to 44% of patients BMI > 35. ALP is elevated up in 35% of diabetic patients. Total protein and serum albumin predominated altered especially in patients with diabetes (18%) (table III).
Nutritional Status
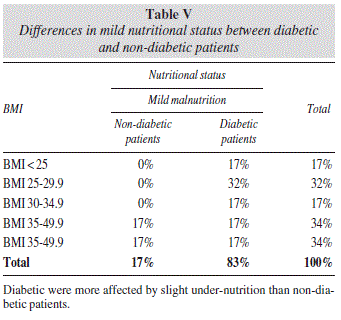
We use the filter tool developed by Ulibarri et al16 to perform nutrition status. We found normal nutrition in 90% of patients and mild malnutrition in 10%. We do not found any moderate and severe malnutrition (table IV).
About mild malnutrition, diabetics have higher percentage than non-diabetic patients (table V).
Discussion
When there are abnormalities in the metabolic pathways can produce different diseases. Therefore, it is important to examine the relationships between alterations of the metabolism that may lead to the development of diseases. The alterations of the metabolic pathways of iron metabolism and liver are associated with the development of insulin resistance and metabolic syndrome. Diseases such as diabetes and obesity are considered difficult to treat because they are intimately linked and could affect the nutritional status.
The purpose of this paper is to know how the iron and the liver metabolism are linked to obesity and diabetes, and how they affect the nutritional status of patients linked to weight gain cumulative type (fig. 1 y 2).
We evaluated 131 patients attended to the clinic in order to underwent to tailored One Anastomosis Gastric Bypass (BAGUA) because of their status of obesity and/or diabetes mellitus. The presence of obesity and/or diabetes in these patients caused comorbidities such as metabolic syndrome, respiratory diseases, polycystic ovary syndrome, anxiety-depressive disorder or complications of diabetes type micro and macro vascular19.
The patients were submitted a series of laboratory studies and the results show big abnormalities in patients with diabetes and greater degree of obesity. These data indicate that increased BMI produces alterations in laboratory studies analyzed. Alterations are predominant in patients BMI > 35 where usually are associated comorbidities mentioned by WHO27.
Abnormal laboratories were most frequently found in the age range of 51 years and older, and predominant in male gender. The patients with diabesity clustered higher percentage of altered laboratory studies.
In non-diabetic patients BMI > 35, 13% showed iron altered, but the percentage increases with the addition of diabetes (26%). We are agree with Fernandez-Real et al8 because of alterations of iron metabolism are directly related to insulin resistance, the metabolic syndrome and diabetes development in the presence of oxidative stress and release of cytokines inflammatory destabilizing the human body.
Vitamin B12 is low in some patients probably by several mechanisms as Kibirige and Mwebaze12 describe, where that deficiency of vitamin B12 is associated with intake of metformin in 5,8 to 33% of patients with T2DM. The 6% of our diabetic patients share these characteristics. The mechanisms of altered vitamin B12 are explained by alterations in small bowel motility with bacterial flora impact, competitive inhibition, inactivation of the absorption, abnormal intrinsic factor receptor, the interaction with cubulin or nutritional status by inadequate intake could determine their deficiency16, and it is closely linked to abnormalities of iron and its consequences.
The components of liver metabolism are proteins that serve as markers for the diagnosis of obesity and liver diseases as fatty liver and others comorbidities, which are important clinical manifestations of metabolic syndrome. Nayeen et al11 demonstrated that the alkaline phosphatase and transaminases are used as predictors for obesity and they are closely related to the amount of lean tissue.
Hsieh et al28 shown that the prevalence of diabetes is higher in patients with liver disease. Transaminases and alkaline phosphatase are elevated in patients with diabetes but in a significantly higher in patients with diabesity. Vozaroba et al29 demonstrated that the presence of high levels of ALT are associated with obesity and insulin resistance with its depletion and the subsequent development of T2DM, therefore suggests using as a predictive marker. The elevated ALT is also associated with increased risk of cardiovascular disease and metabolic syndrome demonstrated by Pirola and Sookoian30.
On the other hand, elevated levels of bilirubin are associated with low prevalence of peripheral arterial disease that are correlated with amputations in diabetic patients as demonstrated by Chan et al31. Have protective effect on the antioxidant properties that prevent cardiovascular disease10. In our study we found that 6% of diabetic patients BMI < 35 have elevated bilirubin. Besides, GGT is predominantly elevated in patients BMI > 35 and this could indicate liver damage, cardiovascular risk and metabolic syndrome as demonstrated by Giannini et al32. Bonnet et al studied 1309 non-diabetic patients for 3 years, where GGT proved to be better predictor of T2DM than ALT9. Our patients with higher BMI had altered GGT and transaminases too.
Hepatic profile has also proteins that reflect the nutritional status16. The 2% of non-diabetic patients and 5% of diabetics with BMI < 35 had elevated total protein. The albumin was elevated in our patients that may reflect liver damage of various etiologies including systemic diseases as mentioned by Giannini et al32.
We determined that certain studies of iron metabolism and liver function have cumulative effects when altered. We realized that with the increase in BMI, there are a greater percentage of patients with altered laboratory studies. However, total bilirubin, iron, albumin and total protein are affected without regard to the gradual increase in BMI.
Huffman et al33 mentioned that there are associations among micronutrient deficiency, diet low in fiber and high saturated fat intake with the deteriorating health of patients with T2DM. He found deficiencies of iron, vitamin and calcium according to different characteristics of the patients.
We use the filter developed by Ulibarri et al16 to determine the degree of nutrition, showing that 10% of total patients have mild malnutrition, but the percentage of diabetic patients with mild malnutrition have almost five times more the than non-diabetic patients.
Patients who achieved higher obesity were shown not to be more affected, possibly because of the adipose tissue expandability described by Lumeng et al34.
Finally, we agree with Soloirzano-Pineda et al13 who mentioned that the high incidence of malnutrition in surgical patients should receive advice of nutritional status with a docketed method and prevent complications.
We are aware that further studies with more patients and longer follow-up are needed to find solutions related to the prevention and treatment of disorders of the metabolic pathways linked to the development of obesity, diabetes or metabolic syndrome.
The main limitation of our study is the number of patients. However, there are no studies about the influence of obesity and/or diabetes linked to weight gain cumulative type, and this may be the watershed to study in depth.
All this new knowledge, help us not only to understand the pathophysiological mechanisms of diseases of fashion, but also to improve surgical techniques bariatric or metabolic rate for the treatment of weight control and long-term improvement or cure of diabetes for surgical treatment.
Conclusions
Iron metabolism in 7% of diabetic patients < 35, and 26% of BMI > 35 was altered. The iron alterations looks like correlated to altered levels of vitamin B12 (lower in 4% of non-diabetics BMI > 35, and higher 6% of diabetics BMI < 35).
In 6% of diabetic patients BMI < 35 had hyperbilirubinemia. The transaminases AST and ALT are elevated in high percentage of patients BMI > 35 but exacerbated on diabetic patients. GGT is raised from 41% (nondiabetics) to 44% (diabetics patients) of BMI > 35. ALP is elevated up in 35% of diabetic compared to non-diabetic patients (10%). Total protein and serum albumin were altered especially in diabetic patients.
The filter tool developed by Ulibarri et al demonstrates that diabetics are more affected with malnutrition than non-diabetic patients.
With these data, we conclude that once the anomalies in different metabolic pathways are present, are closely related to the onset of obesity, metabolic syndrome and diabetes, caused by the cumulative effects of weight gain.
References
1. Li X, Li C, Shang D, Li J, Han J, Miao Y, et al. The implications of relationships between human diseases and metabolic subpathways. PloS one 2011; 6 (6): e21131. [ Links ]
2. Shimizu H, Timratana P, Schauer PR, Rogula T. Review of Metabolic Surgery for Type 2 Diabetes in Patients with a BMI < 35 kg/m2. J Obes 2012; 2012: 147256. [ Links ]
3. Gortmaker SL, Swinburn BA, Levy D, Carter R, Mabry PL, Finegood DT, et al. Changing the future of obesity: science, policy, and action. Lancet 2011; 378 (9793): 838-47. [ Links ]
4. Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. PNAS 2009; 106(36): 15430-5. [ Links ]
5. Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr 2009; 89 (3): 807-14. [ Links ]
6. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999; 104 (6): 787-94. [ Links ]
7. http://www.who.int/en/ (Internet). Switzerland: World Health Organization. (Updated 2013; cited 2013 Jun 17). Available from: http://whqlibdoc.who.int/publications/2006/9241594934_eng.pdf. [ Links ]
8. Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes 2002; 51 (8): 2348-54. [ Links ]
9. Bonnet F, Ducluzeau PH, Gastaldelli A, Laville M, Anderwald CH, Konrad T, et al. Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 2011; 60 (6): 1660-7. [ Links ]
10. Kamisako T, Masuda S, Tanaka Y. Relationship between serum bilirubin and remnant lipoprotein cholesterol level. Clin Lab 2013; 59 (3-4): 435-8. [ Links ]
11. Nayeem F, Anderson KE, Nagamani M, Grady JJ, Lu LJ. Alkaline phosphatase and percentage body fat predict circulating C-reactive protein in premenopausal women. Biomarkers ISO 2010; 15 (8): 663-70. [ Links ]
12. Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab 2013; 12 (1): 17. [ Links ]
13. Soloirzano-Pineda OM, Rivera-Lopez FA, Rubio-Martinez B. Malnutrition incidence in surgical diabetic and non diabetic patients in general surgery department. Nutr Hosp 2012; 27 (5): 1469-71. [ Links ]
14. McGuire S. Shields M, Carroll MD, Ogden CL. Adult obesity prevalence in Canada and the United States. NCHS data brief no. 56, Hyattsville, MD: National Center for Health Statistics, 2011. Adv Nutr 2011; 2 (4): 368-9. [ Links ]
15. Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005; 20 (1): 38-45. [ Links ]
16. de Ulibarri Perez JI, Gonzalez-Madrono Gimenez A, Gonzalez Perez P, Fernandez G, Rodriguez Salvanes F, Mancha Alvarez-Estrada A, et al. New procedure for the early detection and control of hospital malnutrition. Nutr Hosp 2002; 17 (4): 179-88. [ Links ]
17. Wang YC, Gortmaker SL, Sobol AM, Kuntz KM. Estimating the energy gap among US children: a counterfactual approach. Pediatrics 2006; 118 (6): e1721-33. [ Links ]
18. Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med 2010; 61: 393-411. [ Links ]
19. Garciacaballero M, Valle M, Martinez-Moreno JM, Miralles F, Toval JA, Mata JM, et al. Resolution of diabetes mellitus and metabolic syndrome in normal weight 24-29 BMI patients with One Anastomosis Gastric Bypass. Nutr Hosp 2012; 27 (2): 623-31. [ Links ]
20. Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. The N Engl J Med 2012; 366 (17): 1567-76. [ Links ]
21. Fried M, Ribaric G, Buchwald JN, Svacina S, Dolezalova K, Scopinaro N. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI < 35 kg/m2: an integrative review of early studies. Obes Surg 2010; 20 (6): 776-90. [ Links ]
22. Reis CE, Alvarez-Leite JI, Bressan J, Alfenas RC. Role of bariatric-metabolic surgery in the treatment of obese type 2 diabetes with body mass index < 35 kg/m2: a literature review. Diabetes Tech Therapeut 2012; 14 (4): 365-72. [ Links ]
23. Lebovitz HE. Metabolic Surgery for Type 2 Diabetes with BMI <35 kg/m(2): An Endocrinologist's Perspective. Obes Surg 2013; 23 (6): 800-8. [ Links ]
24. Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson B, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012; 367 (8): 695-704. [ Links ]
25. Sauerland S, Angrisani L, Belachew M, Chevallier JM, Favretti F, Finer N, et al. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc 2005 Feb; 19 (2): 200-21. Epub 2004 Dec 08. PMID: 15580436. [ Links ]
26. Garciacaballero M, Martinez-Moreno JM, Toval JA, Miralles F, Minguez A, Osorio D, et al. Improvement of C peptide zero BMI 24-34 diabetic patients after tailored one anastomosis gastric bypass (BAGUA). Nutr Hosp 2013; 28 (Supl. 2): 35-46. Epub 2013 Aug 31. PMID: 23834045. [ Links ]
27. Obesity: preventing and managing the global epidemic. Report of a wHo consultation. World Health Organ Tech Rep Ser 2000; 894: i-xii, 1-253. [ Links ]
28. Hsieh PS, Hsieh YJ. Impact of liver diseases on the development of type 2 diabetes mellitus. World J Gastroenterol: WJG 2011; 17 (48): 5240-5. [ Links ]
29. Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002; 51 (6): 1889-95. [ Links ]
30. Sookoian S, Pirola CJ. Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol 2012; 18 (29): 3775-81. [ Links ]
31. Chan KH, O'Connell RL, Sullivan DR, Hoffmann LS, Rajamani K, Whiting M, et al. Plasma total bilirubin levels predict amputation events in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2013; 56 (4): 724-36. [ Links ]
32. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ 2005; 172 (3): 367-79. PubMed PMID: 15684121. [ Links ]
33. Huffman FG, Vaccaro JA, Zarini GG, Biller D, Dixon Z. Inadequacy of micronutrients, fat, and fiber consumption in the diets of Haitian-, African- and Cuban-Americans with and without type 2 diabetes. Int J Vitam Nutr Res 2012; 82 (4): 275-87. [ Links ]
34. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121 (6): 2111-7. [ Links ]
![]() Correspondence:
Correspondence:
Manuel Garcíacaballero.
Full Professor of Surgery.
University of Malaga.
Medical Faculty.
29080 Málaga. Spain.
E-mail: gcaballe@uma.es
Recibido: 21-I-2014.
Aceptado: 11-II-2014.