Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.29 no.5 Madrid Mai. 2014
https://dx.doi.org/10.3305/nh.2014.29.5.7288
ORIGINAL / Síndrome metabólico
Glycemic and lipid metabolic disorders in diabetic and non-diabetic patients bmi < 35 or > 35 before gastric bypass
Trastornos del metabolismo glicémico y lipídico en pacientes diabéticos y no diabéticos con IMC < 35 ó > 35 antes de bypass gástrico
Manuel Garciacaballero1, Alexander Reyes-Ortiz1,2,3, José Manuel Martínez-Moreno1 and José Antonio Toval-Mata1
1 Dept. of Surgery. Medical Faculty. Malaga. Spain.
2 Autonomous University of the State of Mexico.
3 U.M.F. 229, Del. 16. Mexican Social Security Institute. Mexico.
ABSTRACT
Introduction: Obesity and diabetes are diseases with high prevalence worldwide. There is currently no effective medical treatment for combat the weight gain. It is precursor of diseases such as diabetes or metabolic syndrome. It is necessary to know if weight gain has cumulative effects on the glycemic and lipid metabolism as precursors of complications or comorbidities.
Patients and methods: We studied 131 patients (78 nondiabetic and 53 diabetic), 37 BMI < 35 (3 BMI < 25, 18 BMI 25-29.9, 16 BMI 30-34.9) and 94 BMI > 35 (81 BMI 35-49.9 and 13 BMI > 50). We analyzed BMI, gender, diabetes and the time of evolution. Lipid profile, glucose, HbA1c and C-peptide evaluated after 12-hour fasting.
Results: Diabetic and diabese patients showed high triglycerides. Non-diabetics have impaired glucose (58% BMI < 35 and 36% BMI > 35). The 20% of non-diabetics BMI < 35 had high C-peptide, and 19% of BMI > 35 had high levels. The 5% of diabetics BMI < 35 had low C-peptide and 36% of BMI > 35 had high levels. HbA1c was higher in 40% of non-diabetic patients BMI < 35 compared to 13% BMI > 35.
Conclusions: Glucose and triglycerides increase with age and years of development of T2DM. Age of 51 and more, and men are more affected. The weight increase has cumulative effect by altering the metabolism favoring the onset of diabetes and comorbidities. Despite having intensive control treatment of diabetes, it continues its deleterious effects on patients through the years.
Key words: Obesity. Diabetes mellitus. Diabesity. BMI. Metabolism.
RESUMEN
Introducción: La obesidad y la diabetes son enfermedades de alta prevalencia a nivel mundial. Actualmente no existe un tratamiento médico eficaz para combatir el aumento de peso. La obesidad es precursora de enfermedades tales como la diabetes o el síndrome metabólico. Es necesario saber si el aumento de peso tiene efectos acumulativos sobre el metabolismo de la glucemia y los lípidos como precursores de complicaciones o comorbilidades.
Pacientes y métodos: Se estudiaron 131 pacientes (78 no diabéticos y 53 diabéticos), 37 IMC < 35 (3 IMC < 25, 18 IMC 25-29,9, 16 IMC 30-34,9) y 94 IMC > 35 (81 IMC 3549,9 y 13 de IMC > 50). Se analizó el IMC, el género, la diabetes y su tiempo de evolución. El perfil lipídico, glucosa, HbA1c y el péptido C fueron evaluados después de un ayuno de 12 horas.
Resultados: Los pacientes diabéticos y diabesos mostraron niveles altos de triglicéridos. Los pacientes no diabéticos tienen alteración de la glucosa (58% IMC <35 y 36% IMC > 35). El 20% de los no diabéticos IMC <35 tenía péptido C alto, y un 19% de IMC >35 tenían niveles altos. El 5% de los diabéticos IMC < 35 tenía bajos niveles de péptido C y 36% de IMC > 35 tenían niveles altos. HbA1c fue mayor en 40% de pacientes no diabéticos IMC < 35 frente al 13% de IMC > 35.
Conclusiones: La glucosa y los triglicéridos aumentan con la edad y los años de evolución de la DMT2. La edad de >51 años y los hombres son los más afectados. El aumento de peso tiene efecto acumulativo alterando el metabolismo favoreciendo la aparición de la diabetes y sus comorbilidades. A pesar de tener un tratamiento de control intensivo de la diabetes, esta continúa con sus efectos nocivos sobre los pacientes a través de los años.
Palabras clave: Obesidad. Diabetes mellitus. Diabesidad. IMC. Metabolismo.
Introduction
There is a growing epidemic of chronic diseases related to dietary changes and lifestyle1. Over-weight and obesity are result of excess of energy intake. Fat intake in meals exceeds at least 10% of the daily recommendations2. This can lead to abdominal obesity and insulin resistance, factors that increase 5 times the risk of developing diabetes3.
Obesity is proposed as an inflammatory disease that may develop insulin resistance and type 2 diabetes mellitus (T2DM) as consequence. Obesity has unexpected connections to the risk of develop of cancer, lung diseases, and others diseases. There are a close relationship between excess of nutrients and energy consumption with the alterations in the innate immune response and the cellular and molecular mediators of immunity, where the delicate balance between the immune response and metabolism becomes a pathological relationship4.
World Health Organization (WHO)5 mention that T2DM is a condition with permanent hyperglycemia that increase the risk of micro and macro vascular damage, which is associated with lower quality of life and reduced life expectancy. On the other hand, for the American Diabetes Association (ADA)6, T2DM is a metabolic disorder characterized by hyperglycemia resulting from defects in insulin secretion and/or action associated with damage, dysfunction, and long-term failure of several organs.
Worldwide, 4,8 million of deaths per year were attributed to T2DM. The people with diabetes in 2012 was 371 million and will increase to 552 million in 2030, or one adult in ten7.
In America, the highest prevalence of T2DM is found in the United States of America (USA) and Mexico. The Centers for Disease Control and Prevention (CDC)8 of USA has estimated that 11.3% to 26.9% of U.S. citizens were diabetic in 2011.
The Mexican Diabetes Federation A.A. and the National Survey of Health and Nutrition 2006 (EN-SANUT)9 in Mexico, determined the prevalence of T2DM by 14%. In the population of the Mexican Institute of Social Security (IMSS), the diabetes prevalence was 10,5% for 201010.
In Europe, the prevalence of diabetes by 2010 is diverse. The lowest in Iceland 1.6%, Norway and England 3.6%, Sweden and Ireland 5.2%, Italy 5.9%, France 6.7%, Germany 8.9% and the highest in Cyprus with 9.1%11. In Spain the Diabetes Strategy National Health System12 estimated the prevalence of T2DM in 6,5%.
Obesity is another epidemic disease that could develop T2DM. The increment of 1 kg/m2 increases 9.5% the risk of fasting hyperglycemia, and 8.4% the risk to develop T2DM. The increment of 1 cm in waist circumference increases 3.2% the risk too13.
There are several processes involved in the onset of T2DM as autoimmune destruction of cellular function, decrement by β-pancreatic exhaustion, abnormalities of metabolism of carbohydrate, fat or proteins with insulin resistance, etc.14.
According to the ADA6 the diagnosis is made with FPG ≥ 126 mg/dl (7.0 mmol/l) fasting with at least 8 hours, confirmation is needed with test oral glucose tolerance 2 hours with result ≥ 200 mg/dl (11,1 mmol/l). The International Expert Committee15 recommends glycosylated hemoglobin (HbA1c) too for the diagnosis of diabetes.
The National Institute for Health and Clinical Excellence recommends physical activity; lose weight in order to get healthy BMI, eat diet rich in fiber, fruit and vegetables, and reducing fat of the diet13. ADA and the European Association for the Study of Diabetes (EASD)16 recommend individualize each treatment according to the patient state for which there are several drugs.
Metabolic surgery is now considered as definitive treatment of diabetes, because of the immediate effect that is achieved with the improvement or resolution of T2DM after surgery, where the patient is discharged without antidiabetic treatment. The immediate resolution of T2DM depends on mechanisms due to the change of the gastrointestinal transit17,18.
The obesity prevalence in USA for 2009 was 35,5% for women, and 32.2% for men. The Behavioral Risk Factor Surveillance System (BRFSS) and the CDC19 showed progressive increase of 1.1% of national prevalence of obesity in previous years, so some researchers suggest that by the year 2050, where perhaps 100% of U.S. citizens will have over-weight.
The National Institute of Public Health20 of Mexico for 2012 estimated that over-weight and obesity prevalence is 38,8% and 32,4% respectively.
In Europe, the obesity prevalence is higher in Italy, Portugal, Poland, Czech Republic, Romania and Albania. Eastern Europe and the Mediterranean countries have higher obesity prevalence than Western and Northern Europe21. The consensus SEEDO 2007 of Spain estimated the obesity prevalence as 15,5%22.
The increase in obesity is due to the presence of an obesogenic environment produced by the sedentary lifestyle and dietetic global changes23. Besides, the cognitive aspects of reward and emotional factors play important roles in food intake that may override the homeostatic systems24.
On the other hand, adipose tissue is an active endocrine organ that releases hormones and inflammatory mediators that regulate adipose tissue expansion, control of food intake, energy balance, and others. These effects are involved in the regulation of insulin signaling and lipid metabolism in peripheral tissues that can produce insulin resistance, T2DM or metabolic syndrome23. When the adipose tissue becomes dysfunctional, insulin resistant and dysfunctional lipid storage begins as a sentinel event in the progression of obesity metabolic imbalance4. Each increase of 5 kg of BMI over 25 kg/m2 are associated with 29% increased of total mortality, 41% of mortality associated to vascular disease and 21% of mortality associated with T2DM25.
Treatments for obesity are limited due to the complexity of the disease. Diet and physical activity are effective but adherence is needed26. Behavioral therapy, physical activity, stress management, stimulus control, problem solving, cognitive restructuring, contingency management, and social support are effective too. Specific drugs for obesity are limited and have not been approved for use indefinitely, despite the chronic nature of obesity27.
When treatment fails, weight recovery begins. The safest option for long-term weight loss is through bariatric surgery that provides too resolution of comorbidities19,20, and could protect against the development of T2DM28. Diabetic lean or obese patients, have improvement or remission of their diseases after surgery, secondary to the modification of the gastrointestinal transit29-31.
Patients and methods
Patients
We evaluated 131 patients (78 non-diabetic patients and 53 diabetic), 37 patients BMI ≤ 35 (3 BMI < 25, 18 BMI 25-29,9, 16 BMI 30-34,9) and 94 patients BMI ≥ 35 (81 BMI 35-49,9 and 13 BMI ≥ 50). We analyzed BMI, gender, presence of diabetes and its time of evolution. The laboratory studies were evaluated after at least 12-hour fasting (lipid profile, blood glucose, HbA1c and C-peptide).
BMI was classified as showed in table I. Age was clustered in ranges 15-20, 21-30, 31-40, 41-50, and 51 years of age and older. The duration of T2DM was clustered into 1-5, 6-10, 11-15, 16-20, 21-30 and 31-40 years.
Patients attended medical consultation in order to be undergoing to tailored One Anastomosis Gastric Bypass (BAGUA) for their current state of diabetes, obesity or both conditions.
Surgical procedure
Before surgery, all patients eat only liquid diet during 5 (BMI 23-34) to 7 days (BMI 35-50) depending of preoperative BMI, received antibiotic and antithrombotic prophylaxis. Tailored BAGUA18,31 consisted in the construction of a gastric pouch from the gastroesophageal junction to the end of the minor gastric curvature at the lower level of the cisura angularis. The stapler line of the gastric pouch was fixed approximately 12 cm to the intestinal loop (first layer of the anti-reflux mechanism of BAGUA), and it was anastomosed in a laterolateral position to the mesenteric border of a small bowel loop 100 cm (BMI 23-29), 120 cm (BMI 30-32), 150 cm (BMI 33-34), 200 cm (BMI 35-40), 250 cm (BMI 40-45), and 280 cm distal (BMI 45-50) to the treitz ligament. The anti-reflux mechanism is completed fixing the afferent loop to the gastric remnant and the efferent loop to the antrum.
The size of the gastric pouch depends on the BMI of the patient. In BMI 23-32 we performed a floppy 36 French bougie pouch, while in BMI > 33 a narrow 36 French bougie one s is performed. We left systematically a drainage during the 48 h of hospital stay. After surgery, they eat liquid diet in the 1st week, semifluids in 2nd week, puree in 3-4th weeks and normal diet after one month of surgery. The patient were reviewed at 10 days, 1, 3, 6 and 12 months.
Blood sample processing
Laboratory personnel of the Analysis Unit Clinical Laboratory of University Associated Hospital Parque San Antonio performed the extraction of blood samples from patients with at least 8 hours fasting.
The laboratory samples analyzed were total cholesterol, HDL cholesterol, triglycerides and glucose by ultraviolet-visible spectroscopy. LDL-cholesterol was calculated by Friedman formula. C-peptide by chemiluminescent, and glycated hemoglobin by high performance liquid chromatography (HPLC)/UV-visible detector.
Normal levels of our laboratory are total cholesterol 130-220 mg/dL, HDL cholesterol >35 mg/dL, LDL cholesterol <150 mg/dL, triglycerides 40-160 mg/dL and glucose 65-105 mg/dL.
Statistical analysis
Descriptive statistics was used for comparisons. Differences among groups were analyzed by ANOVA when appropriate, and measures of central tendency according to study variables. Besides, we use percentages on gender, age, BMI, presence or absence of diabetes and its evolution time. For quantitative variables were used mean and standard deviation. To determine the association among explanatory and dependents variables was performed linear regression analysis.
We use SpSS (version 20 for Windows, SPSS, Chicago IL) and Excel 2010 programs.
Results
The 60% were non-diabetic and 40% were diabetic patients. By gender, 51% were women and 49% were men. The predominant age group was ≥ 51 years old (32% of patients). The mean age was 44 ± 13 years. The morbid obesity was predominant. The mean of evolution of T2DM was 13 ± 8 years.
Non-diabetic patients presented triglycerides 161 ± 144 mg/dl, total cholesterol 208 ± 45 mg/dl, HDL cholesterol 48 ± 13 mg/dl, LDL cholesterol 134 ± 44 mg/dl, glucose 97 ± 22 mg/dl, C-peptide 3 ± 1 ng/mL and HbA1c 5 ± 0%.
Diabetic patients presented triglycerides 165 ± 99 mg/dl, total cholesterol 188 ± 40 mg/dl, HDL cholesterol 45 ± 14 mg/dl, LDL cholesterol 112 ± 33 mg/dl, glucose 188 ± 68 mg/dl, C-peptide 2 ± 1 ng/mL and HbA1c 8 ± 1%.
Lipid metabolism
Patients with diabesity were most affected in certain laboratory studies as follow: Triglycerides were higher in diabetic patients (35% of BMI < 35, and 63% of BMI > 35) compared to non-diabetic patients (40% of BMI < 35, and 31% of BMI > 35). The 10% and 34% of nondiabetic patients BMI < 35 and > 35 respectively, had higher total cholesterol compared to 20% and 30% of diabetic patients BMI < 35 and > 35 respectively. Patients with the lowest levels of HDL cholesterol were 12% of non-diabetic compared to 42% of BMI > 35. Once again, the presence of diabetes increases the percentage of patients with LDL cholesterol abnormalities. LDL cholesterol is altered without a tendency to weight gain or adding T2DM.
Lipid profile is altered if the weight or BMI increases as show in figure 1.
As shown in the figure 1, there is a tendency to altered laboratory results with the increment of body weight. This upward trend of lipid profile may explain the vascular complications that may cause higher mortality in these patients.
Glycemic metabolism
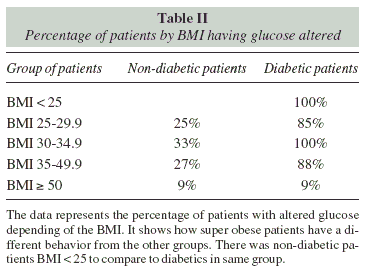
There are high percentage of non-diabetic patients with altered serum glucose (58% of BMI < 35 compared to 36% of BMI > 35). C-peptide were found higher just in non-diabetic patients (2o% of BMI < 35, and 19% of BMI > 35). The 32% of diabetics BMI < 35 had low C-peptide, and 8% had high levels. The 5% of diabetics bMi > 35 had low C-peptide and 36% had high levels.
Glycosylated hemoglobin was found higher in nondiabetic patients BMI < 35 (40%) compared to BMI > 35 (13%), but diabetic patients had higher levels (100% of BMI < 35, and 84% of BMI > 35).
The data obtained showed how the weight gain is closely linked to the onset of metabolic diseases like diabetes.
Table II, show in detail the percentage of glucose alteration according to the level of BMI and the presence or absence of diabetes.
We found a high percentage of non-diabetic patients with hyperglycemia and still no diagnosis of diabetes, therefore it is important that all patients considered as non-diabetics performed serum glucose test in order to prevent the onset of diabetes.
We too found a high percentage of patients with poorly controlled diabetes. Figure 2 shows the mean trend of glycosylated hemoglobin and C-peptide as BMI increases.
By linear regression analysis, we obtained the following results (table III).
The increment of weigh increases the C-peptide, BMI increases total cholesterol, LDL cholesterol and C-peptide. Age increases triglycerides, LDL cholesterol, glucose, C-peptide and glycated hemoglobin. The evolution of T2DM increases the triglycerides and glucose. These data strongly suggest that the addition of factors mentioned in the table III may cause worsening of diabetes.
Discussion
Diabetes and obesity are diseases with high prevalence worldwide. Diabetes can lead to micro and macro vascular complications with significant impact on the patient and society6. The impact of obesity on T2DM, metabolic syndrome and several diseases are important, since they have both physical and psychological effect5,13,27. Worst of all is that there is no medical treatment to eliminate the progression of these diseases.
Both diseases have different effect on the metabolic state if one adds to the other13. It is important to know the functioning of the human body in its different types of metabolism for understanding the health damage, as occur if there is a sustained increase in weight that can impair health12.
There are a variety of treatments for both diseases, but just bariatric surgery offers long-term changes improving both disease and its comorbidities24-27.
The importance of this study is because there is no information about the cumulative effect of the weight on the metabolism of lipids and glycemic with the addition of diabetes.
We evaluated 131 patients attending medical consultation in order to undergo to tailored One Anastomosis Gastric Bypass (BAGUA) because of their obesity and/or T2DM state. The presence of obesity and/or diabetes in these patients caused comorbidities as previously described18.
Subjects underwent a series of laboratory studies and were interpreted and analyzed statistically. These data indicate that increased BMI produces alterations in laboratory studies analyzed. The changes are predominant in patients with BMI > 35 where several comorbidities commonly are associated according to WHO5,7. Suzuki et al24 associate obesity with cardiovascular risk factors and T2DM. The Ontario Health Quality Study in 200527 mention that obesity is associated with higher mortality independent of factors such as smoking. Patients with BMI < 30 are 50% more likely to die than non-obese people are, and the risk is twice from IMC > 35.
We agree with Diabetes Strategy National Health System of Spain 2012 National32, because they mention that obesity increases with age, and men are more affected than women are. As they said, our patients have altered laboratory studies especially in the age range of 51 years and predominantly in male patients. Diabesity patients have higher percentage of involvement.
The percentage of diabetic patients with abnormal lipid profile is higher. From 31% to 63% of patients have hypertriglyceridemia, being most frequent in diabetic and obese patients. The 10% to 60% of patients had higher total cholesterol. From 12% to 45% have higher HDL cholesterol (predominantly in diabesity), and 57% to 100% had lower HDL cholesterol. This data shows higher cardiovascular risk with the increment of BMI as described by Savill13. These patients usually have insulin resistance due to hyperlipidemia as evidenced by Hu33. The CDC (8) mentions that higher BMI produces greater elevation of lipids associated with cardiovascular complications. Bille et al34 found that central obesity is related to higher levels of insulin and triglycerides circulating due to inflammatory role of adipose tissue that causes insulin resistance, ectopic lipid storage and release of dysfunctional inflammatory mediators affecting pancreatic islets, liver, muscle and hypothalamus, causing metabolic dysfunction as evidenced by other studies4,23.
The 32% of non-diabetic patients have hyperglycemia, 19% have higher C-peptide and 15% have elevated HbA1c. This shows undiagnosed diabetes as mentioned by Centers for Disease Control and Prevention8.
In general, the percentage of patients with high levels of C-peptide is higher if BMI increases, probably due to greater body mass that can lead to depletion of insulin production by excessive use of β-pancreatic cell in time, as demonstrated by studies ADA and EASD16.
Almost all diabetic patients have decreased C-peptide and elevated HbA1c. This highlights the higher risk of micro and macro vascular complications on this type of patients as mentioned by the United Nations5.
We found that diabetic patients have a greater increase in triglycerides and serum glucose with decreased C-peptide in relation to non-diabetics. These data confirm that dysfunctional adipose tissue causes hypertriglyceridemia, insulin resistance, hypergly -cemia and decrease of β-pancreatic cell function because of depletion (reduced C-peptide)17. If all these data come together, the human body deteriorate with the development of the known complications. Diabetic patients despite having hypoglycemic treatment, continue with the progression of diabetes, as mentioned by the ADA and EASD16.
Previous data confirmed that weight gain leads to increase of lipid metabolism alteration34.
Patients who achieved morbid obesity were more affected than other kind of obesity, possibly because of the imbalance of adipose tissue expandability as described by Lumeng et al4 capable of triggering the comorbidities known by several mechanisms33. It is impossible to identify obese patients who will have this imbalance, making it better preventing obesity and thereby directly influence the onset of diabetes12-14.
Although the number of our patients is the main limitation of the study, the findings are very interesting and important, and should encourage further research into metabolic pathways, study larger amounts of patients long-term in order to prevent the development of several metabolic diseases.
Physicians are encouraged to advise bariatric or metabolic surgery in a timely manner to eliminate obesity and improvement or resolution of the whole complex of conditions including T2DM actions that can not only extend life, but also improve the quality of life17,18,27,30
Conclusions
The increment of weight produces metabolic disorders. Diabesity is well known that has serious and deleterious effects on the body. Diabesity and an increased duration of T2DM, generate higher percentages of patients with metabolic disorders compared to non-diabetics. The increment of age in years, the increment of weight, presence of diabetes and its time of evolution, produce cumulative alterations in specific metabolisms. There are high percentage of patients without diagnosed diabetes who have hyperglycemia. Although patients have a treatment for diabetes, advancing age and the years of evolution of diabetes, the glucose is rising along with triglycerides, which can influence the deleterious effects of complications on human body. The patients studied, due to its metabolic condition, took the best decision to undergo bariatric surgery for improvement or resolution of their diseases including diabetes mellitus, so it is important to inform the general population the benefits achieved today.
References
1. Pimentel GD, Arimura ST, de Moura BM, Silva ME, de Sousa MV. Short-term nutritional counseling reduces body mass index, waist circumference, triceps skinfold and triglycerides in women with metabolic syndrome. Diabetol Metab Syndr 2010; 2:13. [ Links ]
2. Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obes Rev 2011 May; 12 (5): e95-e106. [ Links ]
3. Rodrigues TC, Canani LH, Gross JL. Metabolic syndrome, insulin resistance and cardiovascular disease in type-1 diabetes mellitus. Arq Bras Cardiol 2010 Jan; 94 (1): 134-9. [ Links ]
4. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011 Jun; 121 (6): 2111-7. [ Links ]
5. http://www.who.int/en/ (Internet). Switzerland: World Health Organization. (Updated 2013; cited 2013 Jan 11). Available from: http://whqlibdoc.who.int/publications/2006/9241594934_eng.pdf. [ Links ]
6. Diagnosis and classification of diabetes mellitus. Diabetes care 2013 Jan; 36 (Supl. 1): S67-74. [ Links ]
7. www.idf.org (Internet) Belgium: International Diabetes Federation. Strategic plan 2013-2015. (Updated 2013; cited 2013 Jan 11). Available from: http://www.idf.org/sites/default/files/attachments/IDF_Strategic_Plan_2013-2015.pdf. [ Links ]
8. www.cdc.gov/ (Internet). Atlanta GA, USA: Centers for Disease Control and Prevention. (Updated 2011; cited 2013 Jan 11). Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [ Links ]
9. http://www.fmdiabetes.org/ (Internet). México DF: Federación Mexicana de Diabetes. (Updated 2013; cited 2013 Jan 11). Available from: http://www.fmdiabetes.org/fmd/pag/diabetes_numeros.php. [ Links ]
10. Gil-Velazquez LE, Sil-Acosta MJ, Dominguez-Sanchez ER, Torres-Arreola Ldel P, Medina-Chavez JH. Practice guideline. Diagnosis and treatment of type 2 diabetes mellitus. Rev Med Inst Mex Seguro Soc 2013 Jan-Feb; 51 (1): 104-19. [ Links ]
11. OECD (2010), "Diabetes Prevalence and Incidence", in OECD/European Union, Health at a Glance: Europe 2010, OECD Publishing. doi: 10.1787/9789264090316-19-en. Available from: http://dx.doi.org/10.1787/9789264090316-19-en (Internet). [ Links ]
12. Ruiz-Ramos M, Escolar-Pujolar A, Mayoral-Sánchez E, Corral-San Laureano F, Fernández-Fernandez I. Diabetes mellitus in Spain: death rates, prevalence, impact, costs and inequalities. Gac Sanit 2006 Mar; 20 (Supl. 1): 15-24. [ Links ]
13. Savill P. Identifying patients at risk of type 2 diabetes. Practitioner 2012 Jul-Aug; 256 (1753): 25-7, 3. [ Links ]
14. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes care 2013 Jan; 36 (Supl. 1): S67-74. [ Links ]
15. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes care 2009 Jul; 32 (7): 1327-34. [ Links ]
16. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetol 2012 Jun; 55 (6): 1577-96. [ Links ]
17. Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012 Apr 26; 366 (17): 1567-76. [ Links ]
18. Garciacaballero M, Valle M, Martinez-Moreno JM, Miralles F, To-val JA, Mata JM, et al. Resolution of diabetes mellitus and metabolic syndrome in normal weight 24-29 BMI patients with One Anastomosis Gastric Bypass. Nutr Hosp 2012 Mar-Apr; 27 (2): 623-31. [ Links ]
19. Yanovski SZ, Yanovski JA. Obesity prevalence in the United States-up, down, or sideways? N Engl J Med 2011 Mar 17; 364 (11): 987-9. [ Links ]
20. ensanut.insp.mx (Internet). México: Centro de Investigación en Nutrición y Salud. Instituto Nacional de Salud Pública. Encuesta Nacional de Salud y Nutrición 2012. (Updated 2013; cited 2013 Jan 11). Available from: http://ensanut.insp.mx/doctos/analiticos/ObesidadAdultos.pdf. [ Links ]
21. Berghofer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC public health 2008; 8: 200. [ Links ]
22. Salas-Salvado J, Rubio MA, Barbany M, Moreno B, Giupo Colaborativo de la S. SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med Clin (Barc) 2007 Feb 10; 128 (5): 184-96; quiz 1 p following 200. [ Links ]
23. Ahima RS. Digging deeper into obesity. J Clin Invest 2011 Jun; 121 (6): 2076-9. [ Links ]
24. Suzuki K, Jayasena CN, Bloom SR. Obesity and appetite control. Exp Diabetes Res 2012; 2012: 824305. [ Links ]
25. Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ 2011 Oct 4; 183 (14): E1059-66. [ Links ]
26. Field BC, Chaudhri OB, Bloom SR. Obesity treatment: novel peripheral targets. Br J Clin Pharmacol 2009 Dec; 68 (6): 830-43. [ Links ]
27. Health Quality O. Bariatric surgery: an evidence-based analysis. Ont Health Technol Assess Ser 2005; 5 (1): 1-148. [ Links ]
28. Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson B, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012 Aug 23; 367 (8): 695-704. [ Links ]
29. Kao YH, Lo CH, Huang CK. Relationship of bypassed limb length and remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2012 Nov-Dec; 8 (6): e82-4. [ Links ]
30. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003 Oct; 238 (4): 467-84; discussion 84-5. [ Links ]
31. Garciacaballero M, Martinez-Moreno JM, Toval JA, Miralles F, Minguez A, Osorio D, et al. Improvement of C peptide zero BMI 24-34 diabetic patients after tailored one anastomosis gastric bypass (BAGUA). Nutr Hosp 2013 Mar; 28 (2): 35-46. [ Links ]
32. www.msssi.gob.es (Internet). España: Ministerio de Sanidad, Servicios Sociales e Igualdad. (Updated 2013; cited 2013 Jan 12). Available from: http://www.msssi.gob.es/organizacion/sns/planCalidadSNS/pdf/excelencia/cuidadospaliativos-diabetes/DIABETES/Estrategia_en_diabetes_del_SNS_Accesi-ble.pdf. [ Links ]
33. Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes care 2011 Jun; 34 (6): 1249-57. [ Links ]
34. Bille DS, Banasik K, Justesen JM, Sandholt CH, Sandbaek A, Lauritzen T, et al. Implications of central obesity-related variants in LYPLAL1, NRXN3, MSRA, and TFAP2B on quantitative metabolic traits in adult Danes. PloS one 2011; 6 (6): e20640. [ Links ]
35. Nayeem F, Anderson KE, Nagamani M, Grady JJ, Lu LJ. Alkaline phosphatase and percentage body fat predict circulating C-reactive protein in premenopausal women. Biomarkers 2010 Dec; 15 (8): 663-70. [ Links ]
![]() Correspondence:
Correspondence:
Manuel Garcíacaballero.
Full Professor of Surgery.
University of Malaga.
Medical Faculty.
29080 Málaga. Spain.
E-mail: gcaballe@uma.es
Recibido: 21-I-2014.
Aceptado: 11-II-2014.