My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.30 n.1 Madrid Jul. 2014
https://dx.doi.org/10.3305/nh.2014.30.1.7322
ORIGINAL / Pediatría
Association between nutritional status, C-reactive protein, adiponectin and HOMA-AD in Brazilian children
Asociación entre estado nutricional, proteína C reactiva, adiponectina y HOMA-AD en niños brasileños
Ana Luiza Gomes Domingos1, George Luiz Lins Machado-Coelho PhD2, Ana Carolina Pinheiro Volp PhD3, Fernando Luiz Pereira de Oliveira PhD4, Ivo Santana Caldas PhD5 and Silvia Nascimento de Freitas PhD3
1Graduate Program in Health and Nutrition. School of Nutrition.
2School of Medicine.
3Department of Social and Clinical Nutrition. School of Nutrition.
4Department of Statistics.
5Research Center in Biological Sciences. Federal University of Ouro Preto (UFOP). Ouro Preto. Minas Gerais. Brazil.
This project was funded by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (APQ- 02851-10) and Federal University of Ouro Preto. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ABSTRACT
Introduction: In children, the presence of obesity is a major risk factor for the occurrence of cardiovascular diseases on the adulthood.
Objective: To evaluate the association of anthropometry, body composition, clinical variables and biochemical profile with C-reactive protein and adiponectin levels, and insulin resistance in children in the municipality of Nova Era, Brazil.
Methods: Nested case-control study following a crosssectional study. We evaluated 178 children, 57 of them classified as obese and 121 as normal-weight from a population of 1024 schoolchildren 6 to 10 years old: Blood samples were collected after 12-hour fast to obtain serum and plasma. We collected anthropometric and body composition measures, systolic and diastolic blood pressure data. Sexual maturation was assessed according to the stage of sexual development. We performed Student's t-test, Mann-Whitney U test, Pearson's correlation, Spearman's test and multiple linear regression analysis. Independent variables with p < 0.05 were included in the multiple regression model. Residual analysis was performed to assess model validity.
Results: Among obese children, C-reactive protein levels were associated with triacylglycerol levels and body fat percentage estimated by skinfold thickness (R2adjusted = 27.6%, p < 0.001). Adiponectin was associated with HOMA-IR, HOMA-AD and body fat percentage estimated by skinfold thickness (R2adjusted = 75.5%, p < 0.001). HOMA-AD index was associated with HOMA-IR, adiponectin, systolic blood pressure and weight (R2adjusted = 90.7%, p < 0.001).
Conclusion: Significant associations were found between body composition, anthropometry, clinical variables, biochemical profile and adiponectin and C-reactive protein levels and insulin resistance in obese and normal-weight children.
Key words: Inflammation. Child. Insulin resistance. Obesity. Biological markers.
RESUMEN
Introducción: En niños, la obesidad es um factor de riesgo para enfermedades cardiovasculares en la edad adulta.
Objetivos: Asociaciar la antropometría, composición corporal, variables clínicas y bioquímicas con la proteína C reactiva (PCR), adiponectina y resistencia a la insulina en niños de Nova Era, Brasil.
Métodos: Estudio de casos y controles anidado en un transversal. Se evaluaron 178 niños, 57 obesos y 121 eutróficos en una población de 1.024 escolares de 6 a 10 años. Las muestras de sangre se recogieron después de 12 horas de ayuno. Recogimos las medidas antropométricas, de composición corporal y presión arterial. La madurez sexual fue evaluada de acuerdo con el desarrollo sexual. Se realizo las pruebas t de Student y U de MannWhitney, las correlaciones de Pearson y Spearman y el análisis de regresión lineal múltiple. Se incluyeron en el modelo de regresión, las variables independientes con p < 0,05. Se realizo el análisis residual para evaluar la validez del modelo.
Resultados: Entre los niños obesos, los niveles de PCR se asociaron con los triglicéridos y el porcentaje de grasa corporal (%GC) estimada por los pliegues cutáneos (R2ajustado = 27,6%, p < 0,001). La adiponectina se asoció con HOMA-IR, HOMA-AD y % GC estimada por los pliegues cutáneos (R2ajustado = 75,5%, p < 0,001). El HOMA-AD se asoció con HOMA-IR, adiponectina, presión arterial sistólica y peso (R2ajustado = 90,7%, p < 0,001).
Conclusiones: Se encontraron asociaciones entre la composición corporal, antropometría, variables clínicas, perfil bioquímico, adiponectina, PCR y la resistencia a la insulina en niños obesos y eutróficos.
Palabras clave: Inflamación. Niño. Resistencia a la insulina. Obesidad. Marcadores biológicos.
Abbreviations
BF: Body Fat.
BIA: Bioelectrical Impedance Analysis.
BMI: Body Mass Index.
BP: Blood Pressure.
C INDEX: Conicity Index.
CRP: C-Reactive Protein.
DP: Standard Deviation.
HDL-C: High-Density Lipoprotein Cholesterol.
HOMA-AD: Homeostatic Model Assessment-Adiponectin.
HOMA-IR: Homeostatic Model Assessment for Insulin Resistance.
IL-1: Interleukin 1.
IL-10: InterleukinlO.
IL-6: Interleukin 6.
LDL-C: Low-Density Lipoprotein Cholesterol.
MUAC: Mid-Upper Arm Circumference.
ND: Not Detected.
SBP: Systolic Blood Pressure.
SST: Subscapular Skinfold Thickness.
TBIA: Tetrapolar Bioelectrical Impedance.
TC: Total Cholesterol.
TFM: Trunk Fat Mass.
TNF-α: Tumor Necrosis Fator-alpha.
TST: Triceps Skinfold Thickness.
VIF: Variance Inflation Factor.
WC: Waist Circumference.
Introduction
Obesity is a chronic multifactorial and complex disease, a low-grade chronic inflammatory state resulting from altered secretion of cytokines, chemokines and hormones1. Studies show that prevalence of overweight and obesity in children has increased significantly in many countries, including Brazil2.
Importantly, excess body fat is a possible risk marker for cardiovascular disease, metabolic syndrome, insulin resistance, and dyslipidemia. It is noteworthy that atherosclerotic process arising from the combination of endothelial dysfunction and inflammation may begin with the development of fatty streaks in childhood3.
It is essential to analyze obesity as an inflammatory state, as research proves its direct association with markers of angiogenesis and inflammation even in children and adolescents4.
Thus, studies associate measures of adiposity and biochemical profile with cytokines that modulate the inflammatory state, such as IL-1, IL-6, IL-10, TNF-α, C-reactive protein (CRP), leptin, resistin and adiponectin even in apparently healthy individuals5. CRP, an acute phase protein produced primarily in hepatocytes, has been used in clinical and epidemiological studies as inflammation marker and risk factor for acute myocardial infarction, ischemic stroke, and death from various cancers and pulmonary diseases6,7.
Even a low-grade inflammatory process is probably related to CRP levels in overweight or obese children8.
Among adipokines, adiponectin stands out as one of the most abundant hormones secreted by adipocytes, and its expression decreases as adipose tissue increases. In addition, adiponectin levels are inversely proportional to insulin resistance, type 2 diabetes, cardiovascular diseases, hypertension, atherosclerosis and triglycerides, and directly proportional to HDL cholesterol levels9.
Adiponectin has antiatherogenic, anti-inflammatory and insulin sensitizer properties. Thus, this hormone protects vascular endothelium against processes involved in the pathogenesis of atherosclerosis and diabetes10. In addition, hypoadiponectinemia may be considered an independent biomarker of metabolic syndrome and atherosclerosis even in overweight young people11.
As nutritional status is correlated with inflammatory markers, studies indicate that weight reduction in children can decrease CRP and increase adiponectin levels12.
Since insulin resistance plays a major role in obesity complications, it should also be investigated for being associated with central adiposity, lipid profile and blood pressure in various age groups13.
From the assumptions mentioned above, this study evaluated associations of body composition, anthropometry, clinical and biochemical variables with adiponectin and C-reactive protein levels and insulin resistance in children aged 6 to 10 years old.
Materials and methods
Study design and subjects
In 2009 we conducted a nested case-control study following a cross-sectional study with 1024 children aged 6 to 10 years old from public municipal schools in Nova Era, state of Minas Gerais, Brazil. In the first stage of the study we found a prevalence of 2.3% underweight, 11.2% overweight and 6.4% obesity by calculating z-score of body mass index for age (BMI-for age)14 (fig. 1).
In the second stage we selected two normal-weight children (n = 130) for each obese child (n = 65) making a total sample of 195 students.
Children with acute, chronic, degenerative diseases, changes in the gastrointestinal tract (e.g. diarrhea), weight loss in the past six months, under special diets, medication that alters metabolism or affects inflammation pathways, showing values of C-reactive protein above 10 mg/L and elite athletes were excluded from the study. Finally we included 57 obese and 121 normal-weight children in the sample.
The study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Federal University of Ouro Preto (2007/93).
Consent was written and signed in duplicate by those responsible.
Anthropometry and blood pressure
Students were instructed not to perform intense workouts before anthropometry and body composition assessment, to attend school with light clothing, not to ingest large volume of water in the 24 hours before the tests, and be fasting for at least 4 hours. All measurements were performed on the right side of the students' bodies.
Weight (Tanita® BC554 Ironman, Illinois, USA) and height (Alturexata®, Belo Horizonte, Brazil) were measured and body mass index (BMI) was calculated by dividing weight by the square of height. Circumferences were measured with a tape measure to the nearest 0.1 cm. Mid-upper arm circumference (MUAC) was measured in duplicate at the midpoint between the acromion process of the scapula and the olecranon. Waist circumference (WC) was measured in triplicate at the midpoint between the anterior superior iliac crest and the last rib, then we calculated the Conicity index (C index)15.
We also measured triceps skinfold thickness (TST) and subscapular skinfold thickness (SST) using a Cescorf® skinfold caliper (Cescorf Equipamentos Ltda, Porto Alegre, Brazil) in triplicate and not consecutively. TST was measured at the midpoint between acromion and olecranon on the posterior arm, and SST on the 45o diagonal line formed by the skinfold, 2 cm below the inferior angle of the scapula. Trunk fat mass (TFM) was obtained with the formula TFM = SST (mm)/TST (mm)16.
Blood pressure (BP) was measured by oscillometry and Doppler ultrasound with Omron® HEM 705 CP and Doppler DV 610. The procedure was performed three times at 2-minute intervals and after a 5-minute rest period before the first blood pressure measurement. Values were replaced by averages.
Body Composition Assessment
Body fat percentage (% BF) was assessed by bipolar bioelectrical impedance analysis (bipolar BIA) using a Tanita® BC554 scale with body fat rate 0.1% and tetrapolar bioelectrical impedance (TBIA) using the Bioscan Maltron® BF-916. We also used an equation to predict body fat percentage by summing triceps and subscapular skinfolds (Body fat from skinfold)17.
Biochemical Assessment
After a 12-hour fast, 10 mL blood was collected from the median cubital vein into disposable tubes, then fractionated in vials either containing sodium fluoride for glucose analysis or no anticoagulant for assessing total cholesterol and its fractions.
Samples were processed using an Excelsa Baby® 206-2 centrifuge (FANEM, São Paulo, Brazil). After centrifugation, serum was aliquoted into three amber microtubes and stored at -80oC for later analysis.
Blood glucose was measured by the enzymatic-colorimetric method and insulin by chemiluminescence. Levels of triglycerides and total cholesterol (TC) were determined in an CM 200 analyzer (WIENER LAB, Rosario, Argentina) by the enzymatic colorimetric method using Triglycerides Liquicolor mono and Cholesterol Liquicolor test kits (Human do Brazil, Itabira, Brazil). High-density lipoprotein cholesterol (HDL-C) was measured by the enzymatic colorimetric direct HDL-PP method (Analisa, Gold Analisa Diagnostica Ltda, Belo Horizonte, Brazil) and levels of Low-density lipoprotein cholesterol (LDL-C) were calculated using the Friedewald equation. Subsequently, the atherogenic index was obtained by dividing Total cholesterol by HDL-C18.
Levels of C-reactive protein were assessed with an Immage® 800 analyzer (Beckman Coulter, Fullerton, California, USA) by nephelometry with a detection limit of 0.1 mg/dL. Adiponectin levels were measured by the sandwich ELISA method in a Human Adiponectin ELISA kit (Liconplex Kit, EZHADP-61K, Linco Research - St Charles Missouri-USA).
Subsequently, we calculated the homeostatic model assessment for insulin resistance (HOMA-IR) from the equation HOMA-IR = (fasting insulin (μUI/mL) x fasting glucose (mmol/mL)/22.5).19 Homeostatic model assessment-adiponectin (HOMA-AD) was obtained by using the formula HOMA-AD = insulinemia (mU/L) x glicemia (mg/dL)/adiponectina (μg/mL).20
Pubertal development
Development stages of the children's pubic hair were analyzed, based on the stages proposed by Tanner. Students were classified as prepubertal (stage 1), pubertal (stages 2, 3 and 4) and postpubertal (stage 5).
Statistical analysis
Identification of probability distributions is relevant, as depending on the distribution that best fits the data set choices concerning which inferential procedures will be applied may vary. In this study the AndersonDarling test was employed. This test examines whether a data sample comes from a population with specific distributions. For the present study, tests were conducted to verify whether the data came from a normally distributed population. A normal distribution was obtained for the samples, whereas for others it was not. Variables were presented as mean ± standard deviation for the samples with normal distribution and median and interquartile range for samples was not. For comparison between groups were performed T-tests and Wilcoxon-Mann-Whitney a test fitting non-parametric data. For this study an α-level of 0.05 was used across all statistical tests.
As dependent variable we consider the concentrations of CRP, adiponectin and HOMA-AD. Tests were performed Pearson correlation and Spearman, to verify the associations between each of the independent variables and the dependent. Multiple linear regression models were estimated, and the independent variables that showed p-value less than 0.05 were considered significant, which were the "biological plausibility" and "epidemiological relevance"21.
Analyses of waste each model in order to check the validity of the assumptions of normality, homoscedasticity and independence between observations. The statistics "Cook's distance" and "Variance Inflation Factor (VIF)", were used to identify outliers and verification of possible multicollinearity of the independent variables. For statistical analyzes we used the statistical software R. 2.13.1.
Results
We evaluated a sample of 178 children, 104 females (58.4%) and 74 males (41.6%). Comparing the two groups of obese and normal-weight children, there were no statistical differences between medians of age, HDL-C, adiponectin, and systolic blood pressure (SBP Omron). However, obese children had significantly higher mean and median values of the other variables compared to normal-weight group (table I).
Sexual maturation assessment based on pubic hair growth 55% of children diagnosed with obesity were prepubescent, 43.3% pubescent and 1.7% postpubescent. Regarding normal-weight children, 66.9% were classified as prepubertal and 33.1% as pubescent. No statistical differences were found between degree of sexual maturation between obese and normal-weight groups (data not shown).
We performed Pearson's correlation and Spearman's correlation tests with independent variables. CRP and adiponectin levels and HOMA-AD index are shown in table II.
Transformations were performed on dependent variables for constructing the final multiple regression model. These changes were necessary to ensure that residual assumptions were met.
Among normal-weight children, variables associated with CRP levels were blood glucose, age, arm circumference, HDL-C and weight (R2adjusted = 16.1%, p < 0.001), whereas in the group of obese children associations occurred with body fat percentage estimated by skinfold thickness and triacylglycerol levels (R2adjusted = 27.6%, p < 0.001) (table III).
In normal-weight children, adiponectin was associated with HOMA-AD (log) HOMA-IR and height (R2adjusted = 87.4%, p < 0.001), whereas in obese children adiponectin levels were associated with HOMA-IR, HOMA-AD (log) and body fat percentage estimated by skinfold thickness (R2adjusted = 75.5%, p < 0.001) (table IV).
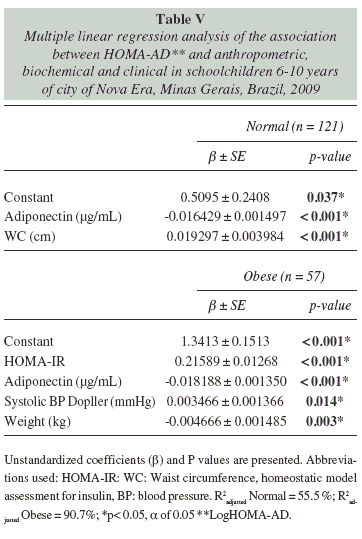
HOMA-AD was associated with adiponectin and waist circumference (R2adjusted = 55.5%, p < 0.001) in normal-weight children. Among obese children, associated independent variables were HOMA-IR, adiponectin, systolic blood pressure (Doppler) and weight (R2adjusted = 90.7%, p < 0.001) (table V).
Discussion
In this study, body composition, anthropometry, biochemical and clinical profiles of normal-weight and obese children were associated with adiponectin, C-reactive protein levels and HOMA-AD.
As noted, CRP levels were associated with blood glucose levels, age, arm circumference, HDL-C, and weight in normal-weight children (R2adjusted = 16.1%, p < 0.001). Among obese children, association was found with triacylglycerol levels and body fat percentage estimated by skinfold thickness (R2adjusted = 27.6%, p < 0.001) thus highlighting the role of adipose tissue in subclinical inflammation.
Progressive increases in body adiposity may result in cellular hypoxia, adipocyte death by necrosis or apoptosis, and increased secretion of cytokines and proinflammatory chemokines, which favors states of insulin resistance. High levels of insulin and blood glucose increase free fatty acid levels, which are associated with high levels of triglycerides and low HDL-C levels22.
CRP has been used in studies with different age groups as a marker of inflammatory processes. In a 2004 study with children and adolescents, Lambert et al. showed that BMI and insulin levels were the main determinants of altered CRP levels. In addition, individuals with the highest quartile of CRP levels had 1.4, 1.7, and 2.3 times higher chance of having high systolic blood pressure, high triglycerides, and low HDL-C levels compared with those with levels below the 75th percentile23.
A study of obese and normal-weight adolescents found a positive association between CRP levels and obesity. Along with the sum of skinfolds, the z-score of BMI explained 42.1% of variation in protein levels24.
Furthermore, another cross-sectional study showed relationship between adipose tissue and inflammation in children and adolescents. Overweight and obese individuals had lower HDL-C and higher triglycerides, CRP, and insulin levels. Thus, the authors concluded that overweight and obese adolescents had higher prevalence of cardiovascular risk factors shown by early signs of atherosclerosis25.
In the present study, we found no statistical difference in adiponectin levels between obese and normal-weight groups. This result can also be explained by the low number of postpubertal children since this stage has significant influence on adiponectin levels, which decrease with sexual maturation26.
However, adiponectin was associated with HOMA-AD, HOMA-IR and height in normal-weight children (R2adjusted = 87.4%, p < 0.001) and HOMA-AD, HOMA-IR and body fat percentage estimated by skinfold thickness in obese children (R2adjusted = 75.5%, p < 0.001).
Through intracellular signaling pathways, adiponectin promotes activation of glucose transport, fatty acid oxidation in muscles, and inhibition of liver gluconeogenesis, which decreases blood glucose levels thus reducing insulin27. In addition, adiponectin inhibits the expression of cell adhesion molecules, scavenger receptors, and inflammatory proteins such as TNFa, IL-6 and IL-128.
A cross-sectional study evaluated obese and overweight children. The authors found that HOMA-IR index, age, and HDL-C were associated with adiponectin levels29. When comparing obese children and adolescents, and their respective controls, Panagopoulou et al in 2008 found that gender and body fat percentage measured by tetrapolar bioimpedance were important determinants of adiponectin levels30.
Reinehr et al. in 2004 evaluated influence of age, gender, puberty, weight loss, and adiponectin levels. The authors concluded that adiponectin levels in obese children were negatively correlated with age, body fat, and insulin resistance, and decreased at puberty. Conversely, significant weight loss increased adiponectin levels and improved insulin resistance10.
In our study, HOMA-AD was associated with adiponectin and waist circumference among normal-weight children (R2adjusted = 55.5%, p < 0.001) and with HOMA-IR, adiponectin, systolic blood pressure, and weight in obese children (R2adjusted = 90.7%, p < 0.001).
Few published studies have used HOMA-AD to assess insulin resistance. However, Makni et al. in 2012 found a significant correlation between HOMA-AD and waist circumference, blood glucose, HDL-C, and blood pressure in obese Tunisian children31.
We emphasized the association of waist circumference with HOMA-AD in normal-weight children. Abdominal obesity, assessed in our study by measuring waist circumference, is associated with visceral fat accumulation, insulin resistance, elevated blood glucose, dyslipidemia, and hypertension32.
Adiponectin levels are inversely proportional to body fat, and low levels promote lower glucose oxidation and increased activation of hormone-sensitive lipase33.
Regarding blood pressure, adiponectin increases nitric oxide production by activating endothelial nitric oxide synthase. Moreover, low adiponectin levels reduce nitric oxide production and vasoconstriction. Also, high insulin levels stimulate endothelin, a powerful vasoconstrictor9.
The present study has some limitations. Our sample consists of children aged 6 to 10 years from a specific municipality, which limits generalizability of results outside this population. In addition, the study design allowed associations to be determined, but not cause-effect results. Therefore, longitudinal studies are needed.
We chose to use self-assessment of sexual maturation without consulting a qualified professional. However, a study by Matsudo & Matsudo has shown moderate to high concordance between the projective technique (Tanner stages) and physician assessment of sexual characteristics34.
Finally, using BMI as a reference method for assessing child obesity at nutritional screening is also a possible limitation of this study. However, several studies have recommended this index as a good tool to predict body adiposity35,36.
Conclusions
In conclusion, our study indicates a significant association of anthropometry, body composition, clinical and biochemical variables with adiponectin and C-reactive protein levels and insulin resistance, as assessed by HOMA-AD in obese and normal-weight children. Thus, we emphasize the importance of early identification of risk factors for cardiometabolic diseases for promoting early intervention and improved quality of life in this population.
Acknowledgements
The authors thank the nutritionists Paula Maria dos Santos and Adriana Cotote Moreira for the assistance in the development of this paper and the Pilot Laboratory of Clinical Analysis of Federal University of Ouro Preto by biochemical analysis.
References
1. Wanderley EN, Ferreira VA. Obesity: a plural perspective. Ciencia & saude coletiva 2010; 15 (1): 185-94. [ Links ]
2. Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa de Orçamentos Familiares 2008-2009. Antropometria e estado nutricional de crianças, adolescentes e adultos no Brasil. (Internet). Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística; 2010. Available from: http://www.ibge.gov.br/home/estatistica/populacao/condicaodevida/pof/2008_2009/POFpublicacao.pdf. [ Links ]
3. Gilardini L, Pasqualinotto L, Di Matteo S, Caffetto K, Croci M, Girola A, et al. Factors associated with early atherosclerosis and arterial calcifications in young subjects with a benign phenotype of obesity. Obesity 2011; 19 (8): 1684-9. [ Links ]
4. Siervo M, Ruggiero D, Sorice R, Nutile T, Aversano M, Iafusco M, et al. Body mass index is directly associated with biomarkers of angiogenesis and inflammation in children and adolescents. Nutrition 2012; 28 (3): 262-6. [ Links ]
5. Wijnstok NJ, Twisk JW, Young IS, Woodside JV, McFarlane C, McEneny J, et al. Inflammation markers are associated with cardiovascular diseases risk in adolescents: the Young Hearts project 2000. The Journal of adolescent health: official publication of the Society for Adolescent Medicine 2010; 47 (4): 346-51. [ Links ]
6. Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010; 375 (9709): 132-40. [ Links ]
7. Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998; 97 (20): 2007-11. [ Links ]
8. Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis 2000; 149 (1): 139-50. [ Links ]
9. Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie 2012; 94 (10): 2143-9. [ Links ]
10. Reinehr T, Roth C, Menke T, Andler W. Adiponectin before and after weight loss in obese children. The Journal of clinical endocrinology and metabolism 2004; 89 (8): 3790-4. [ Links ]
11. Beauloye V, Zech F, Tran HT, Clapuyt P, Maes M, Brichard SM. Determinants of early atherosclerosis in obese children and adolescents. The Journal of clinical endocrinology and metabolism 2007; 92 (8): 3025-32. [ Links ]
12. Tam CS, Clement K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2010; 11 (2): 118-26. [ Links ]
13. Lambert M, O'Loughlin J, Delvin EE, Levy E, Chiolero A, Paradis G. Association between insulin, leptin, adiponectin and blood pressure in youth. Journal of hypertension 2009; 27 (5): 1025-32. [ Links ]
14. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization 2007; 85 (9): 660-7. [ Links ]
15. Valdez R. A simple model-based index of abdominal adiposity. Journal of clinical epidemiology 1991; 44 (9): 955-6. [ Links ]
16. Haffner SM, Stern MP, Hazuda HP, Pugh J, Patterson JK. Do upper-body and centralized adiposity measure different aspects of regional body-fat distribution? Relationship to non-insulin-dependent diabetes mellitus, lipids, and lipoproteins. Diabetes 1987; 36 (1): 43-51. [ Links ]
17. Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, et al. Skinfold equations for estimation of body fatness in children and youth. Human biology 1988; 60 (5): 709-23. [ Links ]
18. Castelli WP. Cholesterol and lipids in the risk of coronary artery disease-the Framingham Heart Study. The Canadian journal of cardiology 1988; 4 (Supl. A): 5A-10A. [ Links ]
19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28 (7): 412-9. [ Links ]
20. Matsuhisa M, Yamasaki Y, Emoto M, Shimabukuro M, Ueda S, Funahashi T, et al. A novel index of insulin resistance determined from the homeostasis model assessment index and adiponectin levels in Japanese subjects. Diabetes research and clinical practice 2007; 77 (1): 151-4. [ Links ]
21. Montgomery DC, Peck EA, Vining GG. Introduction to linear regression analysis, 5rd edn. Wiley, 2012. [ Links ]
22. Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. The Journal of clinical investigation 2011; 121 (6): 2094-101. [ Links ]
23. Lambert M, Delvin EE, Paradis G, O'Loughlin J, Hanley JA, Levy E. C-reactive protein and features of the metabolic syndrome in a population-based sample of children and adolescents. Clinical chemistry 2004; 50 (10): 1762-8. [ Links ]
24. Gobel RJ, Jensen SM, Frokiaer H, Molgaard C, Michaelsen KF. Obesity, inflammation and metabolic syndrome in Danish adolescents. Actapaediatrica 2012; 101 (2): 192-200. [ Links ]
25. Caserta CA, Pendino GM, Alicante S, Amante A, Amato F, Fiorillo M, et al. Body mass index, cardiovascular risk factors, and carotid intima-media thickness in a pediatric population in southern Italy. Journal of pediatric gastroenterology and nutrition 2010; 51 (2): 216-20. [ Links ]
26. Bottner A, Kratzsch J, Muller G, Kapellen TM, Bluher S, Keller E, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. The Journal of clinical endocrinology and metabolism 2004; 89 (8): 4053-61. [ Links ]
27. Miller RA, Chu Q, Le Lay J, Scherer PE, Ahima RS, Kaestner KH, et al. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. The Journal of clinical investigation 2011; 121 (6): 2518-28. [ Links ]
28. Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. American journal of physiology Regulatory, integrative and comparative physiology 2005; 288 (5): R1220-5. [ Links ]
29. Madeira IR, Carvalho CN, Gazolla FM, Pinto LW, Borges MA, Bordallo MA. Impact of obesity on metabolic syndrome components and adipokines in prepubertal children. Jornal de pediatria 2009; 85 (3): 261-8. [ Links ]
30. Panagopoulou P, Galli-Tsinopoulou A, Fleva A, Pavlitou-Tsiontsi E, Vavatsi-Christaki N, Nousia-Arvanitakis S. Adiponectin and insulin resistance in childhood obesity. Journal of pediatric gastroenterology and nutrition 2008; 47 (3): 356-62. [ Links ]
31. Makni E, Moalla W, Lac G, Aouichaoui C, Cannon D, Elloumi M, et al. The Homeostasis Model Assessment-adiponectin (HOMA-AD) is the most sensitive predictor of insulin resistance in obese children. Annales d'endocrinologie 2012; 73 (1): 26-33. [ Links ]
32. Despres JP. Abdominal obesity and cardiovascular disease: is inflammation the missing link? The Canadian journal of cardiology 2012; 28 (6): 642-52. [ Links ]
33. Riestra P, Garcia-Anguita A, Lasuncion MA, Cano B, de Oya M, Garces C. Relationship of adiponectin with metabolic syndrome components in pubertal children. Atherosclerosis 2011; 216 (2): 467-70. [ Links ]
34. Matsudo SMM, Matsudo VKR. Self-assessment and physician assessment of sexual maturation in Brazilian boys and girls: Concordance and reproducibility. American Journal of Human Biology 1994; 6 (4): 451-5. [ Links ]
35. Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007; 120 (Supl. 4): S164-92. [ Links ]
36. Cerrillo I, Fernández-Pachón MS, Ortega Mde L, Valero E, Martín FM, Jáuregui-Lobera I, et al. Two methods to determine the prevalence of overweight and obesity in 8-9 year-old children in Seville, Spain. Nutr Hosp 2012; 27 (2): 463-8. [ Links ]
![]() Correspondence:
Correspondence:
Silvia Nascimento de Freitas PhD.
Federal University of Ouro Preto School of Nutrition.
Department of Social and Clinical Nutrition.
Campus Universitario. Morro do Cruzeiro.
35400-000 Ouro Preto. Minas Gerais. Brazil.
E-mail: freitas@enut.ufop.br
Recibido: 5-II-2014.
1.a Revisión: 17-II-2014.
Aceptado: 30-IV-2014.