My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.30 n.3 Madrid Sep. 2014
https://dx.doi.org/10.3305/nh.2014.30.3.7647
ORIGINAL / Síndrome metabólico
Association between ferritin, high sensitivity c-reactive protein (hsCRP) and relative abundance of Hepcidin mRNA with the risk of type 2 diabetes in obese subjects
Relación entre ferritina, PCRUS y expresion de hepcidina con el riesgo de desarrollo de diabetes mellitus tipo 2 en sujetos obesos
Mónica Andrews Guzmán y Miguel Arredondo Olguín
Laboratorio de Micronutriente. INTA. Universidad de Chile. Chile.
This research was supported by the Diabetes and Endocrinology Society of Chile (SOCHED) and FONDECYT No 1085173 and 1110080.
ABSTRACT
Obesity and Type 2 diabetes mellitus share a strong pro-inflammatory profile. It has been observed that iron is a risk factor in the development of type 2 diabetes. The aim of this study was to evaluate the relationship between iron nutritional status and inflammation with the risk of type 2 diabetes development in obese subjects. We studied 30 obese men with type 2 diabetes (OBDM); 30 obese subjects without diabetes (OB) and 30 healthy subjects (Cn). We isolated peripheral mononuclear cells (PMCs) and challenged them with high Fe concentrations. Total mRNA was isolated and relative abundance of TNF-αIL-6 and hepcidin were determined by qPCR. Iron status, biochemical, inflammatory and oxidative stress parameters were also characterized. OBDM and OB patients showed increased hsCRP levels compared to the Cn group. OBDM subjects showed higher levels of ferritin than the Cn group. TNF-α and IL-6 mRNA relative abundances were increased in OBDM PMCs treated with high/Fe. Hepcidin mRNA was increased with basal and high iron concentration. We found that the highest quartile of ferritin was associated with an increased risk of type 2 diabetes when it was adjusted to BMI and HOMA-IR; this association was independent of the inflammatory status. The highest level of hepcidin gene expression also showed a trend of increased risk of diabetes, however it was not significant. Levels of hsCRP over 2 mg/L showed a significant trend of increasing the risk of diabetes. In conclusion, iron may stimulate the expression of pro-inflammatory genes (TNF-α and IL-6), and both hepcidin and ferritin gene expression levels could be a risk factor for the development of type 2 diabetes. Subjects that have an increased cardiovascular risk also have a major risk to develop type 2 diabetes, which is independent of the BMI and insulin resistance state.
Key words: Inflammatory cytokines. Iron. Hepcidin. Ferritin. Obesity.
RESUMEN
La obesidad y la diabetes tipo 2 comparten un perfil pro-inflamatorio crónico leve. Recientemente ha sido reconocido que el hierro es un factor de riesgo para el desarrollo de diabetes tipo 2. El objetivo de este estudio fue evaluar la relación entre el estado nutricional de hierro y la inflamación con el riesgo de desarrollar diabetes mellitus tipo 2 en sujetos obesos. Estudiamos 30 hombres con obesidad y diabetes mellitus tipo 2 (OBDM); 30 sujetos obesos sin diabetes (OB) y 30 sujetos sanos (Cn). Aislamos células mononucleares periféricas (PMCs) y las desafiamos con altas concentraciones de hierro. Se aisló el RNA y se determinó la abundancia relativa de los RNAm de TNF-αIL-6 y de hepcidina mediante RT-PCR cuantitativo. También se determino el marcadores de la nutrición de hierro, parámetros bioquímicos, inflamatorios y de estrés oxidativo. Los sujetos OBDM y OB mostraron elevados niveles de PCRus comparado con el grupo Cn. OBDM además eran portadores de elevados niveles de ferritina comparado con el grupo Cn. La expresión de TNF-α e IL-6 estuvieron aumentadas en las PMCs de sujetos OBDM que fueron tratadas con altas concentraciones de hierro. Se encontró que el mayor cuartil de ferritina se asoció con un aumentado riesgo de desarrollo de diabetes cuando fue ajustado por BMI y HOMA-IR; esta asociación fue independiente del estado pro-inflamatorio. Los mas altos niveles de expresión de hepcidina tambien mostraron una tendencia a aumentar el riesgo de desarrollo de diabetes, sin embargo éste no fue significativo. Niveles de PCRus por sobre los 2 mg/L mostraron que también tienden a aumentar el riesgo de desarrollo de diabetes mellitus tipo 2. Como conclusión, el hierro puede estimular la expresión de genes pro-inflamatorios (TNF-αIL-6), y tanto la ferritina como el aumento de expresión de hepcidina podrían ser factores de riesgo para el desarrollo de diabetes mellitus tipo 2. Sujetos con un alto riesgo cardiovascular medido por PCRus también muestran un riesgo aumentado de desarrollar diabetes mellitus tipo 2.
Palabras clave: Citoquinas pro-inflamatorias. Hierro. Hepcidina. Obesidad.
Introduction
Type 2 diabetes mellitus (T2D) is a metabolic disease characterized by hyperglycemia, resulting from defects in insulin secretion or insulin action. Many hypotheses have been made regarding the initiation of T2D. Cross-sectional and prospective studies have shown increased levels of acute phase response markers in patients with T2D1. Adipose tissue and its endocrine factors play a pivotal role in the development of chronic low-grade pro-inflammatory state, which is a main characteristic of obesity2.
Elevated circulating levels of acute phase proteins and pro-inflammatory cytokines such as TNFμ and IL-6 are indicators of obesity. In addition, tissues are exposed to an excess of nutrients. Taking this into account, and considering the increased circulating saturated fatty acids, result in a constant stimulus for the synthesis of pro-inflammatory cytokines3. These factors induce the activation of c-jun N-terminal kinase (JNK) and/or the inhibitor of k kinase (IKK), both targets of insulin receptor substrate 1 (IRS1) for serine phosphorylation, which inhibits the insulin receptor signaling cascade4.
• In addition, IKK induction triggers NF-κB activation which up-regulates gene expression of inflammatory mediators, resulting in an increased production of pro-inflammatory cytokines. These cytokines then feed into the inflammatory response and enhance the inhibitory signaling of metabolic pathways5,6.
Micronutrients, especially iron, could participate in the etiopathogenesis of T2D. A systemic iron overload, as the one observed during hereditary hemochromatosis, could contribute to abnormal glucose metabolism and the development of diabetes7. Iron is a transitional metal with strong pro-oxidant activity, resulting in the production of reactive oxygen species that lead to an increase in oxidative stress levels8. There is increasing evidence suggesting that a major consequence of oxidative stress is linked either to the primary or secondary cause of multiple acute or chronic human diseases9. Despite the fact that the mechanism through which iron induces diabetes remains uncertain, epidemiological studies reported a positive association between elevated ferritin levels and the risk of developing T2D10. Ferritin is an iron status marker and reflects the amount of body iron stored in healthy individuals; nonetheless, ferritin is also an acute-phase reactant and its synthesis is up-regulated by infection or inflammation, and cytokines such as IL-6 and IL-1 also induce the expression of ferritin11. Consequently, it has been suggested that the increased ferritin levels in T2D are most likely induced by their elevated inflammatory cytokines.
Iron metabolism is regulated by hepcidin, a 25-aminoacid described as an antimicrobial peptide. Under normal circumstances, hepcidin controls the efflux of iron from duodenal enterocytes and macrophages. This process induces endocytosis and leads to the lysosomal degradation of the ferroportin transporter, where the level of released iron in the bloodstream is reduced12. Under chronic inflammatory conditions such as those observed in T2D, excessive cytokines such as IL-6 have a core function in hepcidin production. IL-6 acts directly on hepatocytes to stimulate hepcidin production13. Furthermore, clinical studies have shown higher hepcidin levels in diabetic patients, which correlated with IL-6 and ferritin levels13,14.
In this study, we evaluated the iron biochemical and nutritional status of obese patients with or without type 2 diabetes mellitus. We associated the risk of type 2 diabetes development with the expression of genes related to inflammation and iron regulation in peripheral mononuclear cells exposed to high iron concentrations evaluating the expression of TNF-α and IL-6.
Methods
Study Subjects
Thirty obese patients with type 2 diabetes (OBDM); thirty obese non-diabetic (OB) patients and thirty healthy (Cn group) individuals were included in this study. All individuals were males over 40 years of age. As for the OBDM and OB group, BMI was higher than 30 kg/mt2 and waist circumference above 102 cm; the BMI of the Cn group was less than 28 kg/mt2 and waist circumference less than 95 cm. OBDM patients with insulin treatment were not included. None of the individuals were taking vitamin and mineral supplements. The protocol has been approved by the ethical committee of the Institute of Nutrition and Food Technology (INTA), and an informed consent form has been obtained from all participants.
Anthropometric examination and blood sampling
All patients were weighed and measured. Consequently, their body mass index (BMI) was calculated, waist circumference was measured and blood pressure was determined. A sample of thirty mL of blood was obtained from the anticubital vein after overnight fasting. Eighteen mL of blood were used to measure biochemical indicators such as: glycemia by glucose oxidase reaction (Dialab, Austria), insulin by radioimmunoassay (Coat-A-Count. Siemens. LA, USA), lipid profile by colorimetric method, and hsCRP by immunoprecipitation in liquid phase (Orion Diagnostica, Espoo, Finland). Hematological and iron nutritional status were determined by measuring hemoglobin (Counter counter, Cell Dyn 1700), serum ferritin (sFn) by Elisa, serum iron (sFe) and total binding iron capacity (TIBC) by colorimetric method, soluble transferring receptor (sTfR) by Elisa kit (Ramco. Texas, USA), and total body iron (TBI) was determined by the formula suggested by Cook et al.15. Twelve mL of blood samples for PMCs isolation were collected with EDTA anticoagulant, and processed within 1-2 h.
Determination of lipid peroxidation (TBARS)
The amount of aldehydes generated by lipid peroxidation was measured by the TBARS reaction, which measures the amount of substances reacting with thio-barbituric acid. Serum samples were incubated at 90o C for 30 minutes after the addition of 1.0 mL of 0.67% thiobarbituric acid in 300 μL of 15% trichloroacetic acid and centrifuged at 1,615 g for 10 minutes at 4o C. Absorbance was determined at 535 nm.
Peripheral Mononuclear Cell Isolation
PMCs were separated by Ficoll-Histopaque gradient sedimentation (1.119: density, Sigma, St. Louis, MO). The mononuclear layer was removed and washed twice in PBS, then adjusted to 40x106 PMCs/mL using RPMI-1640 media with gentamicin. PMCs were incubated in a six-well plate with RPMI-1640 media, and challenged with 5 mM Fe:NTA (1:10) and 40 mM Fe:NTA (1:10) (High Fe) for 20 h. Cells were centrifuged at 1,500 g for 5 min and then washed in PBS. Aliquots were separated for total RNA isolation and afterwards stored at -80o C.
Real Time PCR:
RNA from PMCs was extracted using Trizol Reagent according to product protocol (Invitrogen). The extracted total RNA was treated with RNase-Free DNase Set (Qiagen) according to product instructions. Total RNA concentration was measured by absorption at 260 nm. RNA purity and concentration were checked by determining the OD ratio 260/280 nm using a Biowave II Spectrophotometer. Reverse transcription of RNA (1 mg) was done using an AffinityScrip cDNA Synthesis Kit (Stratagene). Real Time PCR was performed using Brilliant II SYBRä Green QPCR Master Mix (Stratagene) on a Max Prooä System 3000. Glyceraldehyde-3-phosphate dehydrogenase (GADPH) and beta-2-microglobulin (B2M) were used as housekeeping genes.
The primers used were: GADPH: CCAGCAAGA-GCACAAGAGGA and TCAAGGGG-TCTACAT-GGCAA; B2M: GATGCCGCATTTGGATTGGA and TGGAGCAACCTGCTCAG-ATA; Hepcidin: GACACCAGAGCAAGCTCAA and GAAAACA-GAGCCACTGGTCA; IL-6: ATGTCTGAGGCT-CATTCTGC and GCGGCTACATCTTTGGAATC; TNF-α: GTTCCTCA-GCCTCTTCTCCT and ACAA-CATGGGCTACAGGCTT. Each of the primers were used in the PCR reaction to amplify their corresponding gene, and the products were confirmed using agarose gel electrophoresis. The number of mRNA copies of target and housekeeping genes were calculated according to the standard curve method. PCR amplification efficiency of each primer pair was calculated from the slope of the standard curve. Melting curve analysis was constructed to verify the presence of gene-specific amplification and for absence of primer dimers. Agarose gel electrophoresis was performed to test amplicon specificity. Final results were reported according to the Pfaffl method (2001)16 and PMCs from control to basal Fe (5 mM Fe:NTA (1:10)) were used as a control sample.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4 software. Anthropometric and biochemical results were expressed as means ± DS, and non-paramethric variables were presented as geometric mean and range. Differences of anthropometric and biochemical parameters and gene expression among T2DM, OB and C groups were evaluated using One-way ANOVA. mRNA expression was expressed as mean ± SEM. All treatments were compared to control PMCs in basal conditions by One way ANOVA, and a Bonferroni post hoc analysis was performed. For ferritin and hepcidin gene expression analysis, subjects were classified into quartiles according to their serum ferritin concentrations and their fold change in hepcidin gene expression. For hsCRP analysis, subjects were classified according to their levels of hsCRP associated to cardiovascular risk. In logistic regression analyses, the dependent variable corresponded to individuals with T2D and without T2D, meanwhile the independent variables of interest were ferritin and hepcidin expression as well as hsCRP levels. Logistic regression was performed for ferritin and hepcidin gene expression analysis adjusted in first place to BMI; also adjusted to BMI and hsCRP, as well as to HOMA-IR. Finally, the expression analysis was adjusted to BMI, hsCRP and HOMA-IR together. Logistic regression for hs-CRP was adjusted in first place to BMI, and then to HOMA-IR. Finally, hsCRP was adjusted to BMI and HOMA-IR together. STATA 10 software was used for logistic regression analysis. Statistical significance was assigned with p<0.05.
Results
OB and OBDM had higher BMIs and abdominal circumferences compared to control subjects (table I). Basal glycemia was increased in OBDM subjects. In this study, there was no difference observed in the blood lipid profile. OB and OBDM subjects showed higher values of HOMA-IR, reasserting insulin resistance conditions (table I). Serum ferritin and hsCRP were higher in the OBDM group than the OB and Cn groups. Additionally, total body iron was increased in OB and OBDM groups (One-way ANOVA, p<0.01). Despite the fact that no statistical differences were found in hsCRP between OB and Cn subjects, 18/30 obese subjects showed elevated levels of hsCRP (cutoff value over 1 mg/L) compared to 6/30 in the Cn group. Transferrin saturation in OBDM subjects was decreased (One-way ANOVA, p<0.05) and OBDM and OB subjects showed increased total body iron (TBI) (table 2). The levels of TBARS in OBDMs were higher than OB and Cn subjects (One-way ANOVA: p<0.001). There were no differences between the OB and Cn groups.
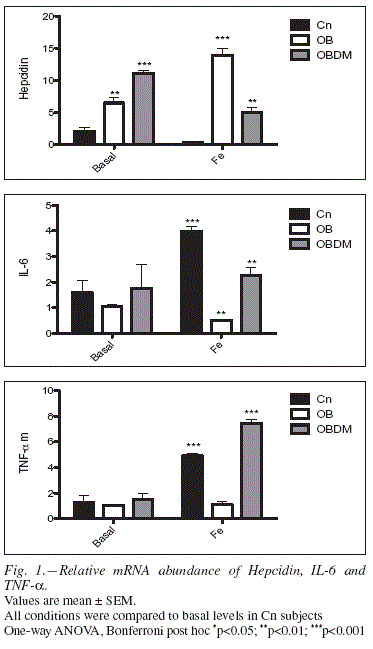
Relative hepcidin gene expression in PMCs of OB and OBDM subjects challenged with high Fe or in basal conditions was higher than in control subjects (figure 1). The relative abudance of IL-6 mRNA was significantly decreased in OB cells challenged with high Fe, but was increased in OBDM subjects (Kruskalwallis, p<0.001). TNF-α relative expression was increased in OBDM cells and in Cn subjects treated with high Fe concentrations. In basal conditions there were no differences (figure 1).
Sample distribution amongst ferritin baseline quartiles, and logistic regression analyses are shown in table III. Compared to the lowest quartiles, subjects in the second, third and fourth quartiles of ferritin had between a 2 and 3-fold increased risk for type 2 diabetes development when the model was adjusted to BMI (p<0.0001). However, when the model was adjusted to BMI and hsCRP, a tendency towards risk increase was shown; nonetheless, it was not significant (p=0.091). When logistic regression was adjusted to HOMA-IR and HOMA-IR, BMI and hsCRP, the risk significantly increased (p=0.014 and p=0.013 respectively).
Subject distribution among fold change in hepcidin gene expression, and the risk of type 2 diabetes mellitus development was also analyzed (table III). The second quartile showed a trend towards being a protecting factor over the risk of type 2 diabetes when adjusted to BMI (OR=0.77, CI=0.13-2.21, p>0.05), HOMA-IR (OR=0.81, CI=0.13-3.82, p>0.05) and to BMI, HsCRP and HOMA-IR (OR=0.62, CI=0.27-10.7, p>0.05). The highest quartile showed a trend towards risk increase; however, it was not significant when it was adjusted to BMI (OR=3.49, CI=0.94-12.90, p>0.05), BMI and hsCRP (OR=3.38, CI=0.85-13.34, p>0.05), and HOMA-IR (OR=3.38, CI=0.79-14.35, p>0.05). However, the adjustment to HOMA-IR, BMI and hsCRP was significative (OR=4.54, CI=0.95-21.66, p<0.05).
Additionally, subjects were distributed according to their levels of hsCRP in order to determine an association with cardiovascular risk. We considered that hsCRP values under 1 mg/L had low cardiovascular risk; meanwhile, levels between 1 and 3 mg/L had moderate risk and values over 3 mg/L were considered as high cardiovascular risk levels [17] (table 4). We observed that the highest cardiovascular risk was also associated with an elevated risk of type-2 diabetes mellitus development, increasing the risk between 3 and 5-fold. The risk was significative in all the performed adjustments: a) to BMI (OR=5.61, CI=0.95-33.07, p<0.032); b) to HOMA-IR (OR=3.16, CI=0.53-18.60, p<0.0004), and c) to HOMA-IR and BMI (OR=3.87, CI=0.61-24.28, p<0.0009).
Discussion
This study focuses on biochemical and iron nutritional parameters of obese subjects with and without type 2 diabetes, as well as its relationship with hepcidin gene expression and oxidative stress parameters such as TBARS. We were also interested in investigating the response of mononuclear cells challenged with high iron concentrations. In OBDM subjects, we observed increased levels of inflammatory parameters such as hsPCR, ferritin and total body iron. On the other hand, we observed decreased transferrin saturation and normal transferrin receptor. These results suggest that an inflammatory state may produce a redistribution of iron in these subjects.
We found that the highest ferritin levels were associated to a risk of type 2 diabetes. However, risk associated to ferritin concentrations depend on an inflammatory state observable after adjusting parameters to hsCRP; the risk of type 2 diabetes related to ferritin levels is also independent to an insulin resistance state. Salonen et al. (1998)17 was first in reporting an association between serum ferritin and the incidence of type 2 diabetes in a group of Finnish men aged 42-60 years. In this study, men with high iron stores - measured as ferritin - were 2.4 times more likely to develop type 2 diabtes compared to men with lower iron stores. This observation was confirmed by a large nested case-control investigation within the Nurse's Health Study (NHS), where an OR for type 2 diabetes was 2.68 after comparing extreme quintiles of ferritin (quintile 5 vs quintile 1), and these results remained statistically significant even after adjustments to CRP levels18.
Our results indicated that hsCRP only increased in OBDM subjects. Although the OB group did not have a significant difference in their hsCRP values compared to the control group, 18/30 had elevated levels of this acute-phase reactant protein, which is an effective predictor of cardiovascular diseases19,20. After analyzing the association between cardiovarcular risk categories for hsCRP and the risk of developing type 2 diabetes, we confirmed that the highest values of hs-CRP had an elevated OR for type 2 diabetes development. This risk was independent of the nutritional and insulin resistance state. This relationship should be further investigated since diabetic patients have high levels of inflammation.
In our work, an increase of IL-6 mRNA in OBDM in PMCs treated with high iron was shown. IL-6 is considered a major proinflammatory cytokine which is produced in a variety of tissues, including activated leukocytes, adipocytes, and endothelial cells5. In models of glucose metabolism in rodents, the infusion of human recombinant IL-6 has shown to induce gluconeogenesis with a subsequent hyperglycemia and compensatory hiperinsulinemia21,22. IL-6 also induces hepatic insulin resistance, which is mediated by an increased expression of SOCS3, a protein that binds and inhibits the insulin receptor, as well as targeting the IRS-1 protein for proteosomal degradation23,24,25,26. The same effect of iron was observed with the mRNA of TNF-α however, in basal conditions the expression of these genes was not increased, demonstrating that iron can trigger an inflammatory response in these kind of cells.
TNF-α and IL-6 can both intensify the insulin resistance state27; yet, we could not demonstrate that the expression of these cytoquines was increased in basal conditions. Since mRNA levels were measured, and therefore protein levels were not analyzed, it is possible that these cytokines may actually be elevated in the bloodstrem and in adition to an increased oxidative stress observed by TBARS. In addition, iron redistribution could explain the insulin resistance state observed in OB and OBDM subjectes through the HOMA-IR index.
In this study TBARs was also associated with the risk of type 2 diabetes (OR=2.91, CI=1.79-4.72, p<0.0001, analysis without adjustment). This association was independent to BMI, hsCRP and HOMA-IR (OR=3.17, CI=1.78-5.64, p<0.0001), indicating that oxidative stress measured by lipoperoxidation has a strong association with type 2 diabetes, which has been previously demonstrated in several studies28,29. Atli et al. (2004)28 found elevated TBARs concentrations in the plasma of type 2 diabetic patients, with normal SOD and GSH-Px activity. Therefore, the increased oxidative stress was not due to reduced antioxidant defense, but rather by an increased free radical production possibly due to hyperglycemia. Furthermore, Song et al. (2007)30 showed that type 2 diabetic subjects had higher levels of TBARS as well as a significant correlation between DNA damage,hyperglycemia,insulin resistance, beta-cell dysfucntion and basal glycemia Additionally, TBARS was correlated with basal glycemia (r=0.40, p<0.0001). These results, together with those of logistic regression, suggest that hyperglycemia could trigger oxidative stress; as well as a nutritional and inflammatory state could enhance oxidative. Nonetheless, tissue damage is independent of these conditions.
The inflammatory state of diabetes mellitus and obesity, together with an increased total body iron, can enhance pathological responses31,32,33,34, particularly those characterized by elevated IL-6 levels. IL-6 may have a core function in hepcidin production35. The binding of IL-6 to its receptor produces the phosphorylation of the intracellular signaling molecule STAT-3. Phosphorylated STAT-3 may dimerize and translocate to the nucleus, where it interacts with an IL-6 response element in the hepcidin gene promoter36. We reported that hepcidin mRNA was up-regulated in cells from OBDM and OB subjects treated with iron. In basal conditions, hepcidin mRNA was also elevated, probably due to the inflammatory state of the subjects. Hepcidin is mainly expressed and synthesized in the liver, although it was recently shown that hepcidin could also be expressed in mononuclear cells37,38. However, the function of hepcidin in this type of cells remains unknown, and its ectopic expression possibly collaborates with the iron redistribution observed in chronic diseases.
Hepcidin expression is also enhanced by increased body iron storage13. The regulation of hepcidin by iron acts as a feedback mechanism allowing sufficient iron to enter the plasma when the demand for iron is high, but also to limit iron release into the plasma in times of iron sufficiency. Therefore, hepcidin acts as a negative regulator of intestinal iron absorption and macrophage iron release13. Hepcidin and ferritin are related to inflammation, both being acute phase proteins. During inflammation, an increase of hepcidin induces a decrease of circulating iron and an increase of ferritin due to inflammation10,12.
We found a trend of association between hepcidin gene expression and the risk of type 2 diabetes. This association depended on the nutritional and inflammatory status, as well as insulin resistance conditions. In the highest quartile of hepcidin expression, the risk of diabetes was between 3-4 fold; hepcidin had a strog relationship with the inflammatory state since it is an acute phase protein. Likewise, the second quartile of hepcidin gene expression showed a trend towards protection over type 2 diabetes risk. On the other hand, when analyzing every component in the hsCRP model, we observed that the subjects had a minor difference between hsCRP, BMI and HOMA-IR levels (data not shown), and these differences were not significant after a One-way ANOVA analysis. Nonetheless, despite these facts, subjects showed an increased risk for cardiovascular disease.
In summary, our results indicated that iron and inflammatory status might interact and at the same time trigger an enhancement of the inflammatory response in OBDM and OB subjects. Inflammation pathways are interconnected, which could be activated by several endogenous and exogenous factors, amongst them, ROS and TNF-α. High levels of ferritin, hepcidin gene expression, hsCRP and TBARS were associated with the risk of developing type 2 diabetes mellitus, and these factors are strongly associated to the nutritional and insulin resistance status.
Conflict of interest
The authors declare that they do not have any conflict of interest.
References
1. Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997; 40:1286-1292. [ Links ]
2. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444:860-867. [ Links ]
3. Dasu M, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptor. Am J Physiol Endocrinol Metab 2010; 300:E145-E154. [ Links ]
4. Wellen K, Hotamisligil G. Inflammation, stress, and diabetes. J Clin Invest 2005; 115:1111-1119. [ Links ]
5. Pradhan A, Manson J, Rifai N, Buring J, Ridker P. C-Reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286:327-334. [ Links ]
6. Gregor M, Hotamisligil G. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 23: 415-445. [ Links ]
7. Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care 2007; 30:1926-1933. [ Links ]
8. Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 2010; 345:91-104. [ Links ]
9. Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs aging 2001; 18:865-716. [ Links ]
10. Ford E.S, Cogswell M.E., Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care 1999; 22:1978-1983. [ Links ]
11. You SA, Wang Q. Ferritin in atherosclerosis. Clin Chim Acta 2005; 357:1-16. [ Links ]
12. Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med 2011;62:347-360. [ Links ]
13. Jiang F, Sun Z, Tang Y, Chuanqing X, Jiao X. Hepcidin expression and iron parameters change in type 2 diabetic patiens. Diabetes Res Clin Pract 2011; 93:43-48. [ Links ]
14. Fernandez-Real JM, Equitani F, Moreno JM, Manco M, Ortega F, Ricart W. Study of circulating prohepcidin in association with insulin sensitivity and changing iron stores. J Clin Endocrinol Metab 2009; 94:982-988. [ Links ]
15. Cook JD, Flowers CH, Skikne BS The quantitative assessment of body iron. Blood 2003; 101:3359-3364. [ Links ]
16. Pfaffl M. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res 2001; 29:2002-2007. [ Links ]
17. Salonen JT, Tuomainen TP, Nyyssonen K, Lakka HM, Punnonen K. Relation between iron stores and non-insulin dependent diabetes in men: case-control study. BMJ 1998; 317: 727-730. [ Links ]
18. Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004; 291: 711-717. [ Links ]
19. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:449-511. [ Links ]
20. Yeh ET. High-sensitivity C-reactive protein as a risk assessment tool for cardiovascular risk. Clin Cardiol 2005; 28:408-412. [ Links ]
21. Stith R, J L. Endocrine and carbohydrate responses to interleukin-6 in vivo. Circulatory Shock 1994; 44:210-215. [ Links ]
22. Tsigos C, Papanicolaou DA, KyrouI, Defensor R, Mitsiadis CS, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab 1997; 82:4167-4170. [ Links ]
23. Sabio G, Das M, Mora A, Zhang Z, Jun J, Ko HJ, Barret T, Kim JK, Davis R. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008; 322:1539-1543. [ Links ]
24. Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furnaletto RW, Mooney RA. Supressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 2003; 278:13740-13746. [ Links ]
25. Torisu T, Sato N, Yoshinga D, Kobayashi T, Yoshioka T, Mori H, Lida M, Yoshimura A. The dual function of hepatic SOCS3 in insulin resistance in vivo. Genes Cell 2007; 12:143-154. [ Links ]
26. Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 2002; 277:42394-42398. [ Links ]
27. Shoelson SE, Lee L, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116:1793-1801. [ Links ]
28. Atli T, Keven K, Avci A, Kutlay S, Turkcapar N, Varli M, Aras S, Ertug E, Canbolat O. Oxidative stress and antioxidant status in elderly diabetes mellitus and glucose intolerance patients. Arch Gerontol Geriatr 2004; 39:269-275. [ Links ]
29. Buyukkocak S, Ozturk HS, Tamer MN, Kacmaz M, Cimen MY, Durak I. Erythrocyte oxidant/antioxidant status of diabetic patients. J Endocrinol Invest 2000; 23:228-230. [ Links ]
30. Song F, Jia W, Yao Y, Hu Y, Lei L, Lin J, Sun X, Liu L. Oxidative stress, antioxidant status and DNA damage in patients with impaired glucose regulation and newly diagnosed type 2 diabetes. Clin Sci 2007; 112:599-606. [ Links ]
31. Sun L, Franco OH, Hu FB, Cai L, Yu Z, Li H, et al. Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly chinese. J Clin Endocrinol Metab 2008; 93:4690-6. [ Links ]
32. Forouhi NG, Harding AH, Allison M, Sandhu MS, Welch A, Luben R, et al. Elevated serum ferritin levels predict new- onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia 2007; 50:949-56. [ Links ]
33. Jehn ML, Guallar E, Clark JM, Couper D, Duncan BB, Ballantyne CM. A prospective study of plasma ferritin level and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 2007; 165:1047-54. [ Links ]
34. Zafon C, Lecube A, Simö R. Iron in obesity. An ancient micro-nutrient for a modern disease. Obes Rev 2009;11:322-328. [ Links ]
35. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004; 113: 1271-6. [ Links ]
36. Fleming RE. Iron and inflammation: cross-talk between pathways regulating hepcidin. J Mol Med 2008; 86:491-4. [ Links ]
37. Zhang X, Robin BH. Hepcidin expression by human monocytes in response to adhesion and pro-inflammatory cytokines. Biochim Piophys Acta 2010; 1800:1262-1267. [ Links ]
38. Pinto JP, Dias V, Zoller H, Porto G, Carmo H, Carvalho F, de Sousa M. Hepcidin messenger RNA expression in human lymphocytes. Immunology 2010; 130:217-230. [ Links ]
![]() Correspondence:
Correspondence:
Dra. Mónica Andrews Guzmán.
Institución INTA,
Universidad de Chile.
El Líbano 5524, Macul,
Santiago, Chile.
E-mail: monica.andrews@gmail.com
Recibido: 30-V-2014.
Aceptado: 15-VII-2014.