Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.30 no.4 Madrid Out. 2014
https://dx.doi.org/10.3305/nh.2014.30.4.7864
ORIGINAL / Cancer
Effect of l-arginine supplementation on insulin resistance and adipocitokines levels in head and neck cancer non diabetic patients after surgery
Efecto de la arginina en la resistencia a la insulina y adipocitoquinas en pacientes con tumores de cabeza y cuello sin diabetes tras cirugia
Daniel de Luis, Olatz Izaola, Beatriz de la Fuente and R. Aller
Center of Investigation of Endocrinology and Nutrition, Medicine School. University of Valladolid. Valladolid. Spain.
ABSTRACT
Introduction: Previous studies have found that L-arginine induced beneficial effects over insulin resistance both in type 2 diabetes mellitus patients and healthy individuals. The aim of our study was to investigate whether an L-arginine enteral supplementation (20 g per day) in head and neck cancer patients could modify insulin resistance, leptin and adiponectin levels after surgery.
Material and Methods: At surgery 82 patients were randomly allocated to two groups: group I received an enteral diet supplements with a high dose of arginine (20g per day) and group II received an enteral formula without arginine. At basal time and on postoperative day 10, the following parameters were recorded: glucose, c-reactive protein, insulin, HOMA (homeostasis model assessment), leptin and adiponectin.
Results: Values of weight, body mass index, fat mass and fat free mass remained unchaged during the acute nutritional intervention in both groups. Insulin levels UI/L (-0.21 +/- 0.18) and HOMA units (-0.07 +/- 0.13) decreased in the arginine group. Adiponectin levels (+1.8 +/- 2.3ng/ml) increased in the arginine group.
Conclusion: Short-term enteral L-arginine therapy addeded to usual enteral nutrition of patients affected by head and neck cancer and surgery without diabetes mellitus type 2 is able to improve insulin resistance and adiponectin levels.
Key words: Adiponectin. Arginine. Head and neck cancer. Insulin resistance. leptin.
RESUMEN
Introducción: Alguntos trabajos han encontrado que la L-arginina induce efectos beneficiosos sobre la resistencia a la insulina, tanto en pacientes con diabetes tipo 2 como en individuos sanos. El objetivo de nuestro estudio fue investigar si la suplementación enteral de L-arginina (20 g por día) en pacientes con cáncer de cabeza y cuello puede modificar la resistencia a la insulina, los niveles de leptina y adiponectina después de la cirugía.
Material y métodos: Tras la cirugía 82 pacientes fueron asignados aleatoriamente a dos grupos: grupo I recibió un enterales suplementos de dieta con una dosis alta de arginina (20 g por día) y el grupo II recibió una fórmula enteral sin arginina. En el momento basal y el día 10 tras la cirugia, se registraron los siguientes parámetros: glucosa, proteína C reactiva, insulina, HOMA (Homeostasis Model Assessment), leptina y adiponectina.
Resultados: Los valores de peso, índice de masa corporal, la masa grasa y la masa libre de grasa se mantuvieron sin cambios durante la intervención nutricional aguda en ambos grupos. Los niveles de insulina UI/L (-0,21 +/-0,18) y HOMA (-0,07 +/-0,13) disminuyeron en el grupo de arginina. Los niveles de adiponectina (1,8 +/-2.3ng/ml) aumentaron en el grupo de arginina.
Conclusión: La nutrición enteral con L-arginina a corto plazo en los pacientes afectados por cáncer de cabeza y cuello y tras cirugía es capaz de mejorar la resistencia a la insulina y los niveles de adiponectina.
Palabras clave: Adiponectina. arginina. Cancer de cabeza y cuello. Resistancia a la insulina. Leptina.
Introduction
The current view of adipose tissue is that of an active secretory organ, sending out adipokines that modulate insulin sensitivity, energy expenditure and inflammation. Adipokines are proteins produced mainly by adipose tissue1. These molecules have been shown to be involved in the pathogenesis of the metabolic syndrome and cardiovascular disease in obese patients. Adiponectin is an adipocyte-derived collagen like protein indentified through an extensive search of adipose tissue. Hypoadiponectinemia increased risk of coronary artery disease togheter with the presence of multiple risk factors, indicating that adiponectin is a key factor of the metabolic syndrome2. Leptin is a protein secreted primarily from adipocytes. Leptin suppresses food intake and increase energy expenditure by enhancing thermogenesis and metabolic rate. Reports suggest that leptin contributes to atheroscleoris and cardiovascular disease in obese patients3. Most studies of serum profile of adipokines have been conducted in cross sectional studies in obese patients4,5 and in dietary intervention trials, which analyzed adipokine changes secondaries to weight loss6,7.
Moreover, nitric oxide, a secondary product of L-arginine, is involved in a wide variety of regulatory mechanisms of the cardiovascular system8. Previous studies have found that L-arginine induced beneficial effects over insulin resistance both in type 2 diabetes mellitus patients and healthy individuals 9,10. Untill now, few data have been available on the effects of L-arginine supplementation in ameliorating insulin resistance and serum adipokines changes after surgery. For example, in cardiopathic nondiabetic patients after an aortocoronary bypass, chronic oral L arginine administration (6.4 g/d) for 6 months increased insulin sensitivity11.
There is evidence that giving patients perioperative nutritional supplements with immunonutritional additives can favourably modulate the immune and inflammatory response both in vitro and in patients with trauma, burns or those undergoing oncological surgery12. Arginine is the most common immunonutrient given to patients with head and neck cancer13. The highest dose of arginine used in these clinical studies was 20 g per day with an improvement in wound complications14. This high dose of arginine administered in these protocols could be used to evaluate the acute effect of a high dose of arginine on insulin resistance and adipocytokines changes induced by cancer surgery.
The aim of our study was to investigate whether an L-arginine enteral supplementation (20 g per day) in head and neck cancer patients could modify insulin resistance, leptin and adiponectin levels after surgery.
Material and methods
Patients and design
The study protocol was designed to study patients with head and neck cancer after a surgery in abcense of type 2 diabetes mellitus or alteration of fasting glucose, diagnosed by fasting plasma glucose less than 110 mg/ dl. Eighty two patients with head and neck cancer eligible for surgery that entered the Departament of Otolaryngology of our Univeristary Hospital participated in this protocol. The study has been approved by the local ethics committee and all patients signed an informed consent. All patients have a histologically proven squamous cell carcinoma of the oral cavity, larynx, oropharyns or hypopharynx and required major ablative surgery. Patients were excluded from the study if they were impaired renal function (serum creatinine concentration > 2.5 mg/ dl), ongoing infections, autoimmune disorders, steroids treatment, nutritional oral supplementation in previous 6 months and severely malnourished (weight loss > 10% of body weight). Baseline studies on all patients consisted of complete history taking and physical examination. General assessment of nutritional status included measurements of height, body weight, body mass index (kg/m2).
Nutritional intervention
Patients were randomly assigned to one of the following treatment groups: group I (42 patients) received an enteral diet supplements with a high dose of L-arginine (20g per day) and group II (40 patients) received an isocaloric, isonitrogenous enteral formula without L-arginine. Blinding of patients and dietitians involved in patient treatment was maintained. Table I shows the composition of the two enteral diets.
Postoperatively, all patients were tubefed for approximately 15 days, as is the standard hospital procedure. The infusion rate was progressively increased every 24 hrs until the daily nutritional goal (35 total kcal/kg; 1.7 g protein/kg) was reached, on postoperative day 4. All patients reached 100% of calculated requirements. No dropouts were present in the study. The end point to discontinuing nutritional support was a minimum oral intake of 1700 cal day and 1 g/kg/d of protein with supplementation with a minimum of 10 days of enteral support.
Biochemical assays
At basal time and on postoperative day 10, the following parameters were recorded: glucose, c-reactive protein (CRP), insulin, HOMA (homeostasis model assessment), leptin and adiponectin.
Plasma glucose levels were determined by using an automated glucose oxidase method (Glucose analyser 2, Beckman Instruments, Fullerton, California). Insulin was measured by enzymatic colorimetry (Insulin, WAKO Pure-Chemical Industries, Osaka, Japan) and the homeostasis model assessment for insulin sensitivity (HOMA) was calculated using these values15.CRP was measured by immunoturbimetry (Roche Diagnostcis GmbH, Mannheim, Germany), analytical sensivity 0.5 mg/dl. Hemoglobine A1c levels were measured by using high-pressure liquid chromatography.
Leptin was measured by ELISA (Diagnostic Systems Laboratories, Inc., Texas, USA) with a sensitivity of 0.05 ng/ml and a normal range of 10-100 ng/ml. Adiponectin was measured by ELISA (R&D systems, Inc., Mineapolis, USA) with a sensitivity of 0.246 ng/ ml and a normal range of 865-21424 ng/ml.
Anthropometric measurements
Body weight was measured to an accuracy of 0.05 Kg and body mass index computed as body weight/ (height2). Waist (narrowest diameter between xiphoid process and iliac crest) and hip (widest diameter over greater trochanters) circumferences to derive waist-to hip ratio (WHR) were measured, too. Tetrapolar body electrical bioimpedance was used to determine body composition16.
Statistical analysis
Sample size was calculated to decrease 10% of insulin resistance with L-arginine administration with 80% power and 5% significance. To minimize the potential for introducing bias, all randomized patients were included in the comparisons, irrespective of whether or not and for how long they complied with their allocated regimen (intention-to-treat analysis). For descriptive purposes, we used median and standard deviation. The distribution of variables was analyzed with Kolmogorov-Smirnov test. Quantitative variables with normal distribution were analyzed with two tailed paired or unpaired Student´s t-test, as needed. Non-parametric variables were analyzed with the Friedman and Wilcoxon tests. A p-value under 0.05 was considered statistically significant. All analyses were performed using SPSS version 15.0 software (SPSS, Chicago, IL).
Results
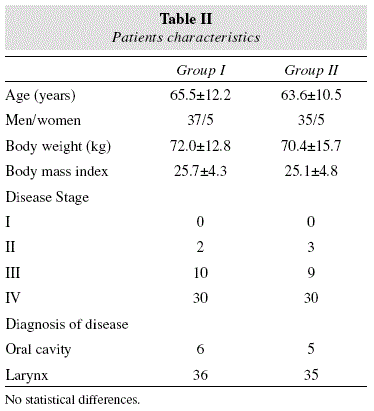
82 patients were enrolled in the study. The mean age was 64.6+11.4 years (10 females (12.2%)/72 males (77.8%)). Basal HbA1c was 4.3+0.5%. There were 42 patients in the group I (high arginine-enhanced formula) and 40 patients in the control diet group II (no arginine-enhanced formula). Characteristics of the patients on enrollment were similar for the two groups, reflecting the homogeneity of the patient population under study. There were no significant intergroup differences with regard to gender, mean age, body weight, location and stage of tumor (table II).
Eleven patients underwent resection of a tumor located in the oral cavity with unilateral or bilateral neck dissection; 71 patients underwent laryngectomy (total or partial) or pharyngo-laryngectomy, with the same distributions of surgery in group I and II (table II).
As shown in table III, no significant intergroup differences in the trend of anthropometric parameters were detected. Values of weight, body mass index, fat mass and fat free mass remained unchaged during the acute nutritional intervention in both groups.
Table IV shows changes in insulin levels, HOMA and adipocitoquines. Insulin levels UI/L (-0.21 +/- 0.18) and HOMA units (-0.07 +/- 0.13) decreased in the arginine group. Adiponectin levels (+1.8 +/- 2.3ng/ml) increased in the arginine group, too.
Discussion
To our knowledge, this is the first study evaluating the effects on insulin resistance, adiponectina and leptin levels of an acute high dose of L-arginine supplementation added on enteral nutrition as usual nutritional treatment in head and neck cancer patients after surgery. In particular, the present study demonstrated that L-arginine supplementation was able to improve both insulin resistance and adiponectin levels.
The beneficial effect of L-arginine on insulin resistance could be mediated by an increase in adiponectin levels, as previously reported 9. The direct effect of adiponectin on atherosclerosis was investigated by Kumada et al17 who showed that adiponectin specifically increased tissue inhibitor of metalloproteinase-1, reducing vascular inflammation and delaying the development of atherosclerosis. With our data, a stimulating result of the present study is that 10 days of L-arginine supplementation at a dose of 20 g/d improved insulin resistance and insulin levels in a group of non diabetic patients submitted to head and neck cancer surgery. This data support a potential beneficial effect of L-arginine on insulin resistance in this type of patients. Previous studies have found that oral L-arginine supplementation improved insulin resistance, endothelial function, oxidative stress in type 2 diabetes mellitus patients 9,18.
As mentioned above, the beneficial effect of oral arginine administration on insulin resistance could be secondary to adiponectin increase. Adiponectin is thought to contribute to the regulation of insulin sensitivity19.
Homeostasis model assessment (HOMA) provides a powerful index of insulin resistance (IR) in epidemiological studies20. Adiponectin and HOMA thus represent two different aspects of IR with opposite relations to this condition. Adiponectin accounts for ~0.02% of total plasma protein in humans, and its concentration is inversely associated with the risk of type 2 diabetes21 and obesity22. Moreover, hypoadiponectinemia is associated with hypertension23, with circulating lipid levels24, and with coronary artery disease25. Consistent with these findings, a chronic inhibition of NO release by N-nitro-L-arginine methyl ester administration in rats induced a state of hypoadiponectinemia, suggesting that NO per se might modulate adiponectin release26 and increasing NO synthesis secondary to oral arginine could modify adiponectin levels.
In a previous study27, after a hypocaloric diet and exercise training program, fasting and postprandial glucose levels were normalized within 3 weeks, in an oral arginine enhanced protocol (8.3 g per day). However, this protocol was performed with obese diabetic patients and with a reduction of caloric intake. Our design showed that the beneficial effect on insulin resistance is only due to arginine because weight remained unchanged during enteral nutrition support. However, this improvement is very small and may not of clinical relevance, without a significant change in glucose levels. Another limitation of this design is the determination of a single isolated determination of the insulin resistance after 10 days of administering arginine. Probably it would have been a need for further determinations, and an analysis of dose effect of arginine. It will therefore be necessary to design new work with different times and concentrations of arginine administered. Another limitation of our work is to use only HOMA as a measure of surrogated insulin resistance, and the impossibility of performing a euglycemic clamp.
In conclusion, short-term enteral L-arginine therapy addeded to usual enteral nutrition of patients affected by head and neck cancer and surgery without diabetes mellitus type 2 is able to improve insulin resistance and adiponectin levels. However, further studies are needed to elucidate if this metabolic changes could be beneficial to this postsurgical population28,29 with an enteral supplementation or to the general population with an oral supplementation regimen.
No conflict of interest
Statement of authorship. All authors have made substantial contributions to all of the following: the conception and design of the study, or acquisition of data, or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content and final approval of the version to be submitted.
Acknowledgements section
D. A de Luis designed the protocol and wrote the article. Olatz Izaola, realized nutritional evaluation. B de la Fuente, realized nutritional evaluation. R Conde, realized laboratory evaluation. D Primo, realized laboratory evaluation. M Gonzalez realized laboratory evaluation.
References
1. Matsuda M, Shimomura I, Sata M. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol C Hem 2002;277:37487-37491. [ Links ]
2. Kumada M, Kihara S, Sumitsuji S. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 2003;23:85-9. [ Links ]
3. Shimomoura I, Hammer RE, Ikemoto S. Leptin reverses insulin resitance and diabetes mellitus in mice with congenital lipodystrophy. Nature 1999;401:73-76. [ Links ]
4. de Luis DA, Aller R, Izaola O, Ovalle HF. The serum profile of adipokines in naïve patients with diabetes mellitus type 2 and obesity. J Clin Lab Anal 2011;25:409-13. [ Links ]
5. De Luis DA, Aller R, Romero E, Dueñas A, Perez Castrillon JL. Relation of phase angle tertiles with blood adipocytokines levels, insulin reisstance and cardiovascular risk factors in obese women patients. Eur Rev Med Pharmacol Sci. 2010 May;14(6):521-526. [ Links ]
6. de Luis DA, Aller R, Izaola O, González Sagrado, Conde R, Perez Castrillon JL. Effects of lifestyle modification on adipocytokine levels in obese patients. Eur Rev Med Pharmacol Sci 2008;12:33-39. [ Links ]
7. de Luis DA, Aller R, Izaola O, Conde R, Gonzalez M, González JM. Effect of a hypocaloric diet in transaminases in no-nalcoholic fatty liver disease and obese patients, relation with insulin resistance. Diab Res and Clin Pract 2008;79:74-78. [ Links ]
8. Böger RH, Ron ES. L-Arginine improves vascular function by overcoming deleterious effects of ADMA, a novel cardiovascular risk factor. Altern Med Rev 2005;10:14-23. [ Links ]
9. Lucotti P, Setola E, Monti LD, et al. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab 2006;291:906-12. [ Links ]
10. Hambrecht R, Hilbrich L, Erbs S, et al. Correction of endothelial dysfunction in chronic heart failure: additional effects of exercise training and oral L-arginine supplementation. J am Coll Cardiol 2000;35:706-13. [ Links ]
11. Lucotti P, Monti L, Setola E, La Canna G, Castiglioni A. Oral L arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metab Cklin and experimental 2009;58:1270-1276. [ Links ]
12. Di Carlo V, Gianotti I, Balzano G, Zerbi A, Braga M. Complications of pancreatic surgery and the role of perioperative nutrition. Dig Dis Sci 1999: 16: 320-326. [ Links ]
13. De Luis DA, Izaola O, Aller R, Cuellar L, Terroba MA (2004): Randomized clinical trial with an enteral arginine-enhanced formula in early postsurgical. Eur J Clin Nutr 58:1505-1508. [ Links ]
14. De Luis DA, Izaola O, Cuellar L, Terroba MC, Martin T, Aller R (2009): High dose of arginine enhanced enteral nutrition in postsurgical head an neck cancer patients. A randomized clinical trial. Eur Rev Med Pharmacol Sci 2009;13:279-283. [ Links ]
15. Mathews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher Df. Homesotasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-414. [ Links ]
16. Picoli A, Brunani A, Savia G. Discriminating between body fat and fluid changes in the obese adult using bioimpedance vector analysi. Int j Obesity 1998;22:97. [ Links ]
17. Kumada M, Kihara S, Noriyuki O, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 2004; 109:2046-9. [ Links ]
18. Wascher TC, Graier WF, Dittrich P, et al. Effects of low-dose Larginine on insulin-mediated vasodilatation and insulin sensitivity. Eur J Clin Invest 1997;27:690-5. [ Links ]
19. Yamauchi T, Kamon J, Waki H, et al. Globular Adiponectin Protected ob/ob Mice from Diabetes and ApoE-deficient Mice from Atherosclerosis. Journal of Biological Chemistry. 2003;278:2461-2468. [ Links ]
20. Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care. 1996;19:1138-1141. [ Links ]
21. Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179-188. [ Links ]
22. Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein,adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79-83. [ Links ]
23. Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179-188. [ Links ]
24. Baratta R, Amato S, Degano C, et al. Adiponectin relationship with lipid metabolism is independent of body fat mass: evidence from both cross-sectional and intervention studies. J Clin Endocrinol Metab. 2004;89:2665-2671. [ Links ]
25. Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85-89. [ Links ]
26. Hattori S, Hattori Y, and Kasai K. Hypoadiponectinemia is caused by chronic blockade of nitric oxide synthesis in rats. Metabolism 54: 482-487, 2005. [ Links ]
27. Lucotti P, Setola E, Monti L, Gallucio E, Costa S, Sandoli E. Beneficial effects of a long term oral L arginine treatment addede to a hypoclaoric diet and exercices training program in obese, insulin-resistanct type 3 diabetes mellitus. AM J Physiol Endocrinol Metab 2006:906-912. [ Links ]
28. Casas-Rodera P., Gomez-Candela C., Benitez S Immunoenhanced enteral nutrition formulas in head and neck cancer surgery: a prospective, randomyzed clinical trial. Nutr Hosp 2008; 23: 105-110. [ Links ]
29. Casas Rodera P, de Luis D A, Gomez Candela C. Immunoenhanced enteral nutrition formulas in head and neck cancer surgery: a systematic review. Nutr Hosp 2012; 27: 681-90. [ Links ]
![]() Correspondence:
Correspondence:
Dr. D. A de Luis
Head of Center of Investigation of Endocrinology and Nutrition.
Medicine Schooll. Valladolid University.
C/ Los perales 16. (Urb Las Aceñas). Simancas.
47130 Valladolid.
Email: mailto:dadluis@yahoo.es
Recibido: 28-VII-2014.
Aceptado: 6-VIII-2014.