Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.30 no.4 Madrid oct. 2014
https://dx.doi.org/10.3305/nh.2014.30.4.7528
ORIGINAL / Otros
Is duration of postoperative fasting associated with infection and prolonged length of stay in surgical patients?
¿Duración del ayuno postoperatorio se asocia con la infección y la estancia prolongada en pacientes quirúrgicos?
Michelli Cristina Silva de Assis1,2,3, Carla Rosane de Moraes Silveira2, Mariur Gomes Beghetto4 and Elza Daniel de Mello1,2
1 Universidade Federal do Rio Grande do Sul. Post-Graduate Program in Medical Sciences, School of Medicine. Porto Alegre, RS, Brazil.
2 Hospital de Clínicas de Porto Alegre. Nutrology Division. Porto Alegre, RS, Brazil.
3 Centro Universitario Unilasalle. Canoas, RS, Brazil.
4 Universidade Federal do Rio Grande do Sul. Department of Assistance and Professional Counseling, Nursing School. Porto Alegre, RS, Brazil.
This study was supported by the Hospital de Clínicas de Porto Alegre Research Incentive Fund.
ABSTRACT
Objective: Verify whether the postoperative fasting period increases the risk for infection and prolonged length of stay.
Methods: Prospective cohort study. Elective surgery patients were included. Excluded: those with no conditions for nutritional assessment, admitted in minimal care units, as well as with <72h in-hospital stay. Postoperative fasting was recorded from the days of no nutrition therapy. The length of stay was considered prolonged when above the average according to the specialty and type of surgery. Logistic regression was used to assess associations and adjust for confounding factors.
Results: 521 patients were analyzed, 44.1% were fasted for a period ≥ 1 day, 91% for ≥ 3 days and 5.6% for more than 5 days. Patients with more than 5 days fasting were more eutrophic, more admitted to intensive care units, and had more postoperative surgical complications. After adjustment for confounding variables, it was noted that > 1 day of postoperative fasting increased the infection risk by 2.04 (CI95%: 1.20 to 3.50), ≥ 3 days 2.81 (CI95%: 1.4-5.8), and in fasting for more than 5 days the infection risk was 2.88 times higher (CI95%: 1.17 to 7.16). The risk for prolonged hospitalization was 2.4 (CI95%: 1.48 to 3.77) among patients who had ≥ 1 day fasting, 4.44 (CI95%: 2.0 to 9.8) and 4.43 times higher (CI95%: 1.73 to 11.3) among patients with ≥ 3 days fasting and more than 5 days, respectively.
Conclusion: The longer duration of postoperative fasting was an independent risk factor both for infection and for prolonged hospital stay.
Key words: Fasting. Surgery. Nutritional status. Infection. Length of stay.
RESUMEN
Objetivo: Constatar si el periodo de ayuno postoperatorio aumenta el riesgo de infección y prolonga la estancia hospitalaria.
Métodos: Estudio de cohorte prospectivo. Fueron incluidos pacientes en cirugía selectiva. Se excluyeron aquellos sin posibilidades de evaluación nutricional, ingresados a unidades de cuidado mínimo y con <72h de estancia hospitalaria. El ayuno postoperatorio fue registrado desde los días sin terapia nutricional. Se consideró que la duración de la estancia hospitalaria era prolongada cuando fue superior al promedio de acuerdo con la especialidad y el tipo de cirugía. Se usó regresión logística para evaluar las asociaciones y ajustarlas a los factores de confusión.
Resultados: Fueron analizados 521 pacientes, un 44,1% ayunó por un periodo de ≥ 1 día, un 91% por ≥ 3 días y un 5,6% por más de 5 días. Pacientes con más de 5 días de ayuno fueron más eutróficos, ingresaron más a unidades de cuidados intensivos y tuvieron más complicaciones quirúrgicas postoperatorias. Después de ajustar las variables de confusión, se encontró que ≥ 1 día de ayuno postoperatorio aumentó el riesgo de infección en 2,04 (CI95%: 1,20 al 3,50), ≥ 3 días 2,81 (CI95%: 1,4-5,8) y en ayunos por más de 5 días el riesgo de infección fue 2.88 veces más alto (CI95%: 1,17 al 7,16). El riesgo de hospitalización prolongada fue 2,4 (CI95%: 1,48 a 3,77) entre pacientes que pasaron por > 1 día de ayuno, 4,44 (CI95%: 2,0 al 9,8) y 4,43 veces más alta (CI95%: 1,73 al 11,3) entre pacientes con ≥ 3 días de ayuno y por más de 5 días, respectivamente.
Conclusión: La mayor duración del ayuno postoperatorio fue un factor de riesgo independiente tanto para la infección como para la estancia hospitalaria prolongada.
Palabras clave: Ayuno. Cirurgía. Estado nutricional. Infección. Tiempo de internación.
Abbreviations list
BMI: body mass index
ASA: American Society of Anesthesiologists
OR: odds ratio
CI: confidence interval
Introduction
Patients who need surgery are subject to mechanisms that may worsen their nutritional status1, such as 1) the primary disease that needs surgical intervention and may initially lead to debilitating states or situations related to ingestion, digestion, and changes in absorption, as well as secondary morbid states (cancer, diabetes, obesity, and other organic chronic dysfunctions)2,3; 2) the surgery in itself, which leads to an increase in the parameters of organic response to stress and may progress into increase in catabolism, consumption of protein mass, and substrate mobilization (protein, fat, and carbohydrates)1,4 and 3) long fasting periods which may lead to a depletion in body reserves, especially protein5.
Many publications in the literature demonstrate that malnourishment is associated to worse in-hospital outcomes6,7, increases in morbidity and mortality rates, and/or higher costs8-10.
The first study that established direct correlation between mortality, postoperative complications, and undernourishment in surgical patients was published by Studley, in 193611.
Studies report prevalence of 20-50% disease-related in-hospital malnourishment12,13. A European study evaluated 5,051 patients admitted in European hospitals using Nutritional Risk Screening-2002, a screening tool, found 32.6% of patients at risk for malnourishment14.
The adoption of prolonged pre and postoperative fasting periods are routine in most surgical centers1. 'Nulla per os' after midnight was introduced before modern surgery and Second World War due to the risk of aspiration during anesthetic induction15. This technique is considered outdated, its foundations being based on the idea that at the moment of anesthetic induction the stomach must be completely empty, thus decreasing the risk for aspiration16. Nevertheless, this period of time is too long from the metabolic standpoint; with starvation (zero protein absorption) and accelerated protein metabolism, there will be negative nitrogen balance, equivalent to a daily loss of 300 to 400 g of lean mass17.
The importance of the deleterious effect of starvation in patients before surgery was reinforced by Joseph Lister more than 100 years ago. 'Nulla per os' regime became universal in spite of contemporary reports that the emptying of clear liquids from the stomach is much faster5,18,19.
In the 80's, it was demonstrated that adequate nutritional preoperative therapy reduced postoperative complications 2.5-fold, postoperative sepsis and mortality six and five-fold, respectively20. Preoperative nutritional interventions based on nutritional status assessment tools prove to be accurate in the selection of surgical patients at nutritional risk21, contributing for the reduction of perioperative morbidity and mortality22.
A guideline about nutrition support in surgery recommends, in general, that food intake should not be interrupted postoperatively, and that oral or enteral feeding should be reestablished within 24 h after the surgery. To avoid increased mortality the indication for immediate postoperative artificial nutrition (enteral or parenteral) also applies to patients with no signs of malnourishment, but who will not receive oral food for more than 7 perioperative days or whose oral food intake will not meet their nutritional needs (i.e. less than 60-80%) for more than 14 days23.
It is recognized that surgical and non-surgical patients admitted in the hospital and at risk of undernourishment who receive nutritional intervention have a shorter length of stay, reduction in length of stay related costs, as well as in complications related costs24,25.
Despite the fact that the aforementioned studies, which include randomized controlled trials, demonstrate the negative effects of decreased nutritional treatment and the importance of early nutritional intervention in the postoperative period, be it enteral or otherwise, in our setting this practice still seems to be little adopted.
Early feeding is already common practice in modern centers, nevertheless this study intended to evaluate, in a tertiary care university hospital in southern Brazil, the time of fasting that elective surgery patients are been submitted to in the postoperative period and whether there is increased risk of adverse hospital outcomes: infection and prolonged hospital stay, adjusting for potential confounders such as age, nutritional status, surgical and medical complications, among others.
Methods
Study design
Prospective observational study developed in a tertiary, high complexity teaching hospital. Each of the subjects signed the informed consent, and the study was approved by the hospital's Ethics and Research Committee (report no 110307).
Population studied
Patients of elective surgery from the following specialties: digestive tract, general surgery, otorhinolaryngology, colon and rectal surgery, thoracic surgery, urology, and vascular surgery were selected from August 1st 2011 to October 25th 2012, at the Hospital de Clínicas de Porto Alegre, Rio Grande do Sul, Southern region of Brazil. The electronic records of the hospital were used to identify eligible patients. The patients who did not have conditions for nutritional assessment were excluded, as well as those admitted to the minimal postoperative care unit, those with a plan of less than 72 hours in-hospital stay, and those admitted for exams. The population studied represented a convenience sample (determined by the availability of the researcher during the study period). The patients who, after inclusion in the study were transferred from the ward to intensive care did not have their nutritional status assessed during that period; nevertheless, they were followed until hospital discharge.
Data collection and variables of interest
The data analyzed were extracted from the records of the care provided by the health practitioners via Hospital de Clínicas de Porto Alegre electronic system.
Demographic data, clinical characteristics, variables of interest, and endpoints were prospectively collected by the researchers in templates on the day of admission or up to 48 hours before the surgical procedures, until the day of hospital discharge or in-hospital death. The clinical characteristics, operative complications, hospital infection, and the days of postoperative fasting were collected daily from the patients' records (electronic and paper).
After inclusion in the study, the patients were assessed for nutritional status by means of: measuring weight and height, arm and calf circumferences, and triceps skin fold, as well as bioimpedance for the assessment of body composition (lean and fat free mass). Nutritional status was classified according to the body mass index (BMI) and a subjective global assessment (SGA). At every 7 days this evaluation was repeated according to the same protocol.
The endpoint infection was collected from notifications by the Infection Control Committee in the electronic system, according to the diagnostic criteria described in this paragraph. For the diagnosis of sepsis a positive blood culture associated to hypotension and hypoperfusion were necessary. Abdominal abscess was defined as the presence of intra-abdominal pus collection with the need for surgical drainage. For urinary tract infection a positive urine culture with more than 100,000 microorganisms was necessary. Pneumonia was documented with an altered chest x-Ray, positive sputum culture, and antibiotics. For wound infection, a positive culture and surgical or spontaneous drainage of pus were necessary.
The endpoint prolonged length of stay was collected as the duration (in days) of hospital stay, and the definition of prolonged was when above the mean or median of 2010 (the year preceding the study), according to the type and size of surgery, as well as the underlying disease that lead to the surgery in each specialty studied.
The exposure variable of interest for the study was postoperative fasting period. Postoperative fasting was counted according to the days that the patient fasted after surgery. The fasting period was not considered when enteral or parenteral nutrition was introduced. Covariables were old age (60 years or more), cancer, surgery classified according to duration (considered small when up to one hour and 30 minutes, medium when between 1 hour and 31 minutes and 3 hours, and large when longer than 3 hours). Admission to intensive care, surgical postoperative complications (needing or not new surgical intervention) such as wound dehiscence or leakage (documented by x-Ray) and clinical complications were also considered as covariables. For medical complications the following diagnostic criteria were used: congestive heart failure was diagnosed according to the standard clinical and radiologic criteria and the need for the treatment with digitalis and diuretics; respiratory failure when mechanical ventilation was needed for more than six hours after the surgery; pulmonary embolism demonstrated by lung scintigraphy or angiography and treated with heparin; stroke documented by a new and persistent neurological deficit.
Statistical analysis
The statistical analysis was done using PASW version 19. Data normality was verified using the Shapiro-Wilk test. Continuous variables with normal distribution were expressed as mean ± standard deviation, and categorical data were expressed as absolute figures or percentages. Comparisons between groups were performed using the chi-square test and Fisher's exact test for categorical variables and Student t-test or Wilcoxon test for continuous variables. Multivariate analysis used a model of binomial logistic regression to calculate the odds ratio (OR) with confidence interval (CI) and adjusted for confounding factors. A two-sided P value less than 0.05 was considered statistically significant. The variables in the logistic regression model were selected from a univariate analysis, considering p<0.20. This model included the variables: age, gender, comorbidities, nutritional status, type of anesthesia, potential surgical contamination, ASA score (American Society of Anesthesiologists), duration of surgery, admission in intensive care, and postoperative surgical and clinical complications.
Results
Patients' characteristics
1,047 patients were selected as potentially eligible during the study period. 383 did not confirm eligibility because they were younger than 18 years of age or had pacemakers (thus rendering electric impedance impossible), had gastroplasty, or were admitted directly to the operating theatre. Consequently, 664 patients were included, of which 143 did not have surgery during hospital stay; 521 subjects remaining for analysis (Figure 1).
Medical and demographic characteristics of the sample studied are listed in Table I. Patients who had 5 or more days of fasting had a higher percentage of cancer, diabetes, and chronic obstructive pulmonary disease. According to the classification of nutritional status by the BMI, there were more undernourished, overweight, and obese patients in the group that did not have 5 or more days of fasting, while patients with 5 or more days of fasting were more eutrophic. There was no difference in nutritional status at admission among patients with a fasting period longer than 5 days, according to the SGA. Patients with 5 or more days of fasting were usually those from digestive tract or colorectal surgeries, were more often admitted to intensive care, and had more surgical postoperative complications.
Fasting postoperative incidence and risk for infection and prolonged hospitalization
Table II depicts the incidence of infection (2nd column) according to each of the exposure variables studied. In the univariate analysis, the outcome infection was significantly associated with longer postoperative fasting: greater than or equal to 1 day (OR=4.1; CI95%: 2.6-6.4), 3 days (OR=6.9; CI95%: 3.7-12.8) and more than 5 days (OR=8.4; CI95%: 3.87-18.75). Infection was also associated with the variables: major surgery, cancer, malnourishment, moderate malnourishment, contaminated, and potentially contaminated surgery, as well as postoperative complication with OR ranging from 6.7 to 2.3 (3rd column of table II). After adjusting for these variables, using multivariate analysis, we found that the risk for infection in patients under fasting greater than or equal to 1 day is 2 times higher (CI 95%: 1.2-3.5) . Longer fasting periods increase this risk even further: greater than or equal to 3 days (OR= 2.8; CI95%: 1.4-5.7) and greater than 5 days (OR=2.9; CI95%: 1.2-7.2). All variables included in the model, except cancer, malnourishment and moderate malnourishment remained conferring risk for infection with OR ranging from 4.3 to 1.2 (Figure 2).
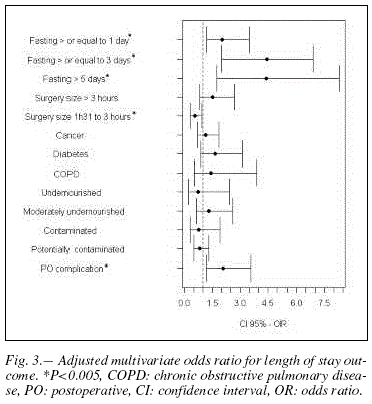
Table II (5th column) shows the incidence of prolonged hospitalization according to the exposure variables studied. In the univariate analysis the endpoint prolonged hospitalization was significantly associated with postoperative fasting period: greater than or equal to 1 day (OR=3.5; CI95%: 2.4-5.1), 3 days (OR=8,7; CI95%: 4.3-7.5) and more than 5 days (OR=8.2; CI95%: 3.7-20.2). Prolonged length of stay was also associated with the variables: major surgery, diabetes, chronic obstructive pulmonary disease, potentially contaminated surgery and postoperative complication with OR that ranged from 3.3 to 1.5 (6th column of table II). After adjusting for these variables, using multivariate analysis, we found that the risk for prolonged length of stay in patients with greater than or equal to 1 day fasting is 2.4 times higher (CI95%: 1.5-3.8). The longer fasting time also increases the risk for prolonged length of stay: greater than or equal to 3 and 5 days, the risk is 4.4 times higher (Figure 3). The variable included in the model which remained conferring risk for prolonged length of stay was postoperative surgical complication (OR=2.1; CI95%: 1.2-3.6) (Figure 3).
Discussion
The present study demonstrated that surgical patients on postoperative fasting for a period equal or exceeding 1 day have a higher risk for infection, adjusting for other confounding variables. The longer the fasting period, the greater the risk for infection. Factors also significantly associated to infection in our study were large surgeries, contaminated surgeries, and the presence of surgical postoperative complications. Likewise, in a review study, risk factors for surgical complication (wound dehiscence) were infection and obesity26, and in the study by Poveda et al., the size of the surgery was also associated to infection27.
The patients who had 1 or more days of fasting also presented higher risk for prolonged length of stay, even after adjusting for the other variables. Postoperative complication was also significantly associated to prolonged stay, likewise, in a retrospective cohort study, the patients submitted to colorectal laparoscopy presented less complications and shorter length of stay28.
Such findings confirm the need for early nutritional intervention in the postoperative period to minimize the occurrence of such outcomes29.
A study conducted in elderly surgical patients demonstrated that the implementation of a protocol reducing preoperative fasting from 15 to 4 hours and postoperative food introduction in up to 5 days lead to a decrease in the number of days in the hospital, as well as in infection rates25.
Other authors have also described the association between receiving early and sufficient nutritional intervention (60% of target in proteins and calories) to a reduction in length of stay, as well as hospital costs, in adult surgical patients30,31.
In this study we noted that prolonged postoperative fasting is more frequent among digestive tract surgery and colorectal surgery patients. This practice, traditionally adopted, is based on the fear of causing postoperative complications if feeding begins before intestinal function resumes32. A review of 15 studies comparing 1,352 patients of elective colorectal surgery concluded that early feeding was safe. The complication rate was 12.5% in 935 patients fed early, with no increase in the risk for leaks, aspiration pneumonia or bowel obstruction33.
The patients included in the study who were considered undernourished or moderately undernourished at admission by the SGA had a higher risk for infection in the univariate analysis. This finding is consonant with prospective trials published in the literature6,8,9. In the multivariate analysis, after adjusting for the other variables, even a 1-day fasting period implicated in higher risk for worse outcomes.
Our study demonstrates that the practice of postoperative fasting, still applied in our setting, is significantly associated to infection and prolonged hospitalization, which reinforces the importance of standardized protocols for the use of early enteral or parenteral nutrition according to the disease and surgical procedure.
The absence of randomization, control and sampling group for convenience, which may have led to the inclusion of different types of patients, can be considered as limitations of this study, as well as the lack of information about the calories and proteins ingested, since this was not drawn from the study objective. The evaluation of the preoperative fasting period, especially for patients who underwent gastrointestinal surgery, was not intended to be an initial objective of the study, therefore it may be seen as a limitation, considering the studies16,20,34 which report this period as also crucial for postoperative recovery.
Conclusion
The incidence of postoperative fasting was high and longer periods represented an independent risk factor after adjustment for confounding variables, for infection and prolonged length of stay. These data indicate to health professionals and multidisciplinary teams in nutritional therapy the importance of modern protocols that include the shortening of postoperative fasting according to the nutritional status of surgical patients and type of surgery they are submitted to.
References
1. Abunnaja S, Cuviello A, Sanchez JA. Enteral and parenteral nutrition in the perioperative period: state of the art. Nutrients. 2013;5(2):608:23. [ Links ]
2. Butters M, Straub M, Kraft K, Bittner R. Studies on nutritional status in general surgery patients by clinical anthropometric, and laboratory parameters. Nutrition. 1996;12(6): 405-10. [ Links ]
3. Weimann A, Ebener C, Holland-Cunz S, Jauch KW, Hausser L, Kemen M, et al. Surgery and transplantation - Guidelines on Parenteral Nutrition, Chapter 18. GMS Ger Med Sci 2009;7:Doc10. [ Links ]
4. Bozzetti F. Nutritional support in oncologic patients: where we are and where we are going. Clin Nutr 2011;30(6):714-7. [ Links ]
5. Henriksen MG, Hessov I, Dela F, Vind Hansen H, Haraldsted V, Rodt SA. Effects of preoperative oral carbohydrates and peptides on postoperative endocrine response, mobilization, nutrition and muscle function in abdominal surgery. Acta Anaesthesiol Scand 2003;47(2):191-9. [ Links ]
6. Jensen GL, Mirtallo J, Compher C, Dhaliwal R, Forbes A, Grijalba RF, et al. International Consensus Guideline Committee. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clin Nutr 2010;29(2):151-3. [ Links ]
7. The Prague declaration: stop disease-related malnutrition. (Accessed April 10, 2013 at http://www.european-nutrition.org/images/uploads/pub-pdfs/pdf_pdf_66.pdf). [ Links ]
8. Álvarez-Hernández J, Vila MP, León-Sanz M, de Lorenzo AG, Celaya-Pérez S, García-Lorda P, et al. Prevalence and costs of malnutrition in hospitalized patients; the PREDyCES® Study. Nutr Hosp 2012;27(4):1049-59. [ Links ]
9. Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 2003;22(3):235-9. [ Links ]
10. Pérez de la Cruz A, Lobo Tamer G, Orduna Espinosa R, Mellado Pastor C, Aguayo de Hoyos E, Ruiz Lopez MD. Malnutrition in hospitalized patients: prevalence and economic impact. Med Clin (Barc) 2004;123(6):201-6. [ Links ]
11. Studley HO. Percentage of weight loss. A basic indicator of surgical risk in patients with chronic peptic ulcer. 1936. Nutr Hosp 2001;16(4):141-3. [ Links ]
12. Waitzberg DL, Caiaffa WT, Correia MITD. Hospital malnutrition: the brazilian survey (IBRANUTRI): a study of 4000 patients. Nutrition. 2001;17(7-8): 573-80. [ Links ]
13. Mello ED, Beghetto MG, Teixeira LB, Luft VC. Desnutrição hospitalar: cinco anos após o IBRANUTRI. Rev Bras Nutr Clin 2003;18(2):65-9. [ Links ]
14. Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krähenbühl L, Meier R, et al. EuroOOPS: An international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr 2008;27(3):340-9. [ Links ]
15. McLeod R, Fitzgerald W, Sarr M, for Members of the Evidence Based Reviews in Surgey Group. Preoperative fasting for adults to prevent perioperative complications. Can J Surg 2005;48(5):409-11. [ Links ]
16. Waitzberg DL, Aguilar-Nascimento JE, Correia MITD, Bicudo-Salomão A. Nutrição em cirurgia. In: Waitzberg, DL. Nutrição Oral, Enteral e Parenteral na prática clínica. 4a ed. São Paulo: Atheneu; 2009;1707-28. [ Links ]
17. Dalal KS, Rajwade D, Suchak R. "Nill per oral after midnight": Is it necessary for clear fluids?. Indian J Anaesth 2010;54(5):445-7. [ Links ]
18. Oliveira KGB, Balsan M, Oliveira SS, Aguilar-Nascimento JE. A abreviação do jejum pré-operatório para duas horas com carboidratos aumento o risco anestésico? Rev Bras Anestesiol 2009;59(5):577-84. [ Links ]
19. Mullen JL, Buzby GP, Matthews DC, Smale BF, Rosato EF. Reduction of operative morbidity and mortality by combined preoperative and postoperative nutritional support. Ann Surg 1980;192(5):604-13. [ Links ]
20. Mullen JL, Gertner MH, Buzby GP, Goodhart GL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg 1980;139(1):160-7. [ Links ]
21. Mullen JL, Gertner MH, Buzby GP, Goodhart GL, Rosato EF. Implications of malnutrition in the surgical patient. Arch Surg 1979;114(2):121-5. [ Links ]
22. Weimann A, Braga M, Harsanyi L, Laviano A, Ljunggvist O, Soeters P, et al. ESPEN (European Society for Parenteral and Enteral Nutrition). Nutrition: Surgery including organ transplantation. Clin Nutr 2006; 25(2):224-44. [ Links ]
23. Neumayer LA, Smout RJ, Horn HGS, Horn SD. Early and sufficient feeding reduces length of stay and charges in surgical patients. J Surg Res 2001;95(1):73-7. [ Links ]
24. Dalziel K, Segal L. Time to give nutrition interventions a higher profile: cost-effectiveness of 10 nutrition interventions. Health Promot Int 2007;22(4):271-83. [ Links ]
25. Sandy-Hodgetts K, Carville K, Leslie GD. Determining risk factors for surgical wound dehiscence: a literature review. Int Wound J 2013; doi:10.1111/iwj.12088. [ Links ]
26. Poveda VB, Galvão CM, Hayashida M. Análise dos fatores de risco relacionados à incidência de infeqção do sitio cirúrgico em gastrocirurgias. Rev Esc Enferm USP 2003;37(1):81-9. [ Links ]
27. Kang CY, Chaudhry OO, Halabi WJ, Nguyen V, Carmichael JC, Stamos MJ, et al. Outcomes of laparoscopic colorectal surgery: data from the Nationwide Inpatient Sample 2009. Am JSurg 2012; 204(6):952-7. [ Links ]
28. Bozzetti F. Peri-operative nutritional management. Proc Nutr Soc 2011;70(3):305-10. [ Links ]
29. Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointes Surg 2009;13(3):569-75. [ Links ]
30. Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhance recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 2010;29(4):434-40. [ Links ]
31. Mulrooney LJ, Bagley JS, Wilkinson JA, et al. Nasojejunal feeding does not improve clinical outcomes and is poorly tolerated following colorectal surgery. Proc Nutr Soc 2005;64(Suppl 1):9A. [ Links ]
32. Ng WQ, Neill J. Evidence for early oral feeding of patients after elective open colorectal surgery: a literature review. J Clin Nurs 2006;15(6):696-709. [ Links ]
33. Aguilar-Nascimento JE, Dias ALA, Dock-Nascimento DB, Correia MITD, Campos ACL, Portari-Filho PE, et al. Actual preoperative fasting time in Brazilian hospitals: the BIGFAST multicenter study. Ther Clin Risk Manag 2014;10:107-12. [ Links ]
![]() Correspondence:
Correspondence:
Michelli Cristina Silva de Assis, RN, PhD.
Hospital de Clínicas de Porto Alegre.
Serviço de Nutrologia.
Rua Ramiro Barcelos, no 2350, sala 635.
Bairro Rio Branco, Porto Alegre/RS - Brazil - ZIP: 90035-003.
E-mail: michellicassis@gmail.com; mcassis@hcpa.ufrgs.br
Recibido: 24-IV-2014.
1.a Revisión: 18-V-2014.
2.a Revisión: 19-VI-2014.
Aceptado: 25-VI-2014.