Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.33 no.2 Madrid Mar./Abr. 2016
https://dx.doi.org/10.20960/nh.103
TRABAJO ORIGINAL / Obesidad y síndrome metabólico
Influence of the unsaturated fatty acids on body weight, glucose, and lipids metabolism in obese women with Pro12Pro genotype in PPARγ2 gene
Influencia de los ácidos grasos insaturados en el peso corporal y en el metabolismo de la glucosa y de los lípidos en mujeres obesas con el genotipo Pro12Pro en el gen PPARγ2
Márcia Fófano do Lago1, Vanessa Chaia Kaippert2, Débora Lopes Souto3 and Eliane Lopes Rosado4
1,3,4Department of Nutrition and Dietetics and 2Nutrition Evaluation Laboratory. Federal University of Rio de Janeiro. Institute of Nutrition Josué de Castro. Rio de Janeiro, Brazil
Financial support: CNPq and FAPERJ.
ABSTRACT
Background: The type of dietary fatty acid may have different effects on obesity and its complications, however, these effects can be influenced by genes and polymorphisms, such as peroxisome proliferator-activated receptor γ isoform 2 (PPARγ2). Moreover, it is unclear whether the degree of unsaturation of the fat has different effects on lipid and glucose metabolism, and particularly the loss of body weight.
Objective: To evaluate the influence of diets rich in polyunsaturated fatty acids (PUFA) and monounsaturated fatty acids (MUFA) on anthropometric and biochemical variables in obese woman with genotype Pro12Pro on PPARγ2 gene on body weight, glycemic and lipemic profile.
Methods: Eighteen obese women with Pro12Pro genotype in PPARγ2 gene were randomized into groups to receive a high PUFAs (PUFA-diet, n = 8) or MUFAs (MUFA-diet, n = 10) diets. Anthropometrics (body mass index [BMI] and waist circumference) and biochemical variables (glucose, insulin, HOMA-IR, total cholesterol, LDL-cholesterol, and HDL-cholesterol and triglycerides) were evaluated at baseline and after 45 days.
Results: Anthropometric and biochemical variables were similar between groups at baseline and after intervention (p > 0.05). BMI decrease only in PUFA-diet (p = 0.01), probably due to the lower lipid content in this diet. MUFA-diet decrease fasting glucose (p = 0.03), insulin (p = 0.03), and HOMA-IR (p = 0.02).
Conclusion: Compared to PUFA, MUFA was more efficient to reduce the insulin resistance in obese women with Pro12Pro genotype in PPARγ2, even in high saturated fatty acids and total fat diet.
Key words: Obesity. PPARγ2. Unsaturated fatty acids. Body weight. Insulin resistance. Lipids metabolism.
ABSTRACT
Introducción: el tipo de ácido graso de la dieta presenta diferentes efectos sobre la obesidad y sus complicaciones, pero estos efectos pueden verse influenciados por los genes y sus polimorfismos, tales como los receptores activados por el proliferador de los peroxisomas isoforma γ2 (PPARγ2). Además, no está claro si el grado de insaturación de los lípidos posee diferentes efectos en el metabolismo de los lípidos y de la glucosa y, particularmente, en la pérdida de peso.
Objetivos: evaluar la influencia de dietas ricas en ácidos grasos poliinsaturados (AGPI) y monoinsaturados (AGMI) en las variables antropométricas y bioquímicas en el peso corporal y el perfil glucémico y lipémico en mujeres obesas con el genotipo Pro12Pro en el gen PPARγ2.
Métodos: dieciocho mujeres obesas con genotipo Pro12Pro fueron distribuidas aleatoriamente para una de las dietas, rica en AGPI (n = 8) o AGMI (n = 10). Las variables antropométricas (índice de masa corporal [IMC] y circunferencia de la cintura) y bioquímicas (glucosa, insulina, HOMA-IR, colesterol total, LDL-colesterol, HDL-colesterol y triglicéridos) fueron evaluadas antes y después de un periodo de 45 días.
Resultados: las variables antropométricas y bioquímicas fueron similares entre los grupos antes y después de la intervención (p > 0,05). El IMC disminuyó después de la ingesta de AGPI (p = 0,01), probablemente debido al menor contenido de lípidos. El AGMI redujo la glucosa (p = 0,03), insulina (p = 0,03) y HOMA-IR (p = 0,02).
Conclusión: los AGMI fueron más eficientes para reducir la resistencia a la insulina en mujeres obesas con el genotipo Pro12Pro en el gen PPARγ2, aunque las mujeres presentaran una elevada ingesta de lípidos totales y ácidos grasos saturados.
Palabras clave: Obesidad. PPARγ2. Ácidos grasos insaturados. Peso corporal. Resistencia a la insulina. Metabolismo de lípidos.
Introduction
Obesity results from an imbalance between energy intake and energy expenditure, and may be defined as a disease in which excess body fat has accumulated (1). Obesity increases the risk of cardiovascular disease and has been strongly associated with dyslipidemia and insulin resistance (2).
Body weight is determined by an interaction between genetic, environmental and psychosocial factors acting through the physiological regulation of energy intake and energy expenditure (2).
Several candidate genes have been associated with human obesity. Among these genes, there is the peroxisome-proliferator-activated receptor (PPAR) that is a member of the nuclear hormone receptor superfamily. The predominant isoform of PPAR is the γ2 (PPARγ2), which is expressed selectively and at higher level in adipose tissue, where it modulates the expression of target genes implicated in adipocyte differentiation and glucose homeostasis. A point mutation found on the B exon of the NH2-terminal of PPARγ2, substituting alanine for proline at position 12 (PPARγ Pro12Ala SNP) (rs1801282), has been shown to decrease receptor activity (3). The Pro12Ala polymorphism of the PPARγ2 isoform has an Ala12 allele frequency of around 0.12 in Caucasians, and this variant may contribute to the observed variability in body mass index (BMI), and was associated with greater insulin sensitivity and a more favorable lipid profile (4). Therefore, genetic influences increase the risk of weight gain but they are not sufficient to explain the development of obesity. Other factors are implied in obesity such as lifestyle, dietary habits and environment (1).
Different types of fatty acids display different metabolic behaviors, such as oxidation and deposition rate differences, that may contribute to body weight change (5).
Polyunsaturated fatty acids (PUFAs) may be classified in n-3 fatty acids and n-6 fatty acids. The predominant n-6 fatty acid is arachidonic acid, which is converted to prostaglandins, leukotrienes and other lipoxygenase or cyclooxygenase products (important regulators of cellular functions with inflammatory, atherogenic and prothrombotic effects). The n-3 fatty acids are docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are competitive substrates for the enzymes and products of arachidonic acid metabolism that antagonize the pro-inflammatory effects of n-6 fatty acids (5).
Dietary linoleic acid (the long-chained n-6; 18:2) is converted to arachidonic acid, which serves as a precursor for prostaglandins. A metabolite of these prostaglandins (5-deoxy-D12, 14-prostaglandin J2) was shown to stimulate the differentiation of preadipocytes into adipocytes through its interaction with the PPARγ2. Another prostaglandin (prostaglandin F2α) blocks adipogenesis through activation of the mitogen-activated protein kinase, resulting in inhibition of adipocyte gene expression PPARγ2 (6).
The beneficial properties of n-3 PUFAs have been observed in populations consuming large amounts of cold-water fish (e.g., salmon and tuna), vegetable oils (e.g., soybean and canola), nuts (e.g., walnuts), and seeds (e.g., flaxseed). Foods that contain n-6 PUFAs include other vegetable oils (e.g., corn and sunflower, and sesame), cereal grains, meat, milk, and eggs (5).
The dietary linoleic acid (ALA, a dietary n-6 polyunsaturated fatty acid) may be associated with a reduction of the ratio total cholesterol and HDL. Dietary intakes of PUFA n-3 fatty acids induced changes in lipid metabolism by decreasing triacylglycerol concentrations, and may reduce the fraction of atherogenic small and dense LDL, even in the absence of LDL lowering (5).
Dietary intakes of PUFA may also affect glucose metabolism. PUFA n-6 fatty acids may modulate cytokine production or the release of the soluble tumor necrosis factor alpha receptors through eicosanoid-independent pathways, affecting insulin signal transduction processes (5).
In the past, monounsaturated fatty acids (MUFA) were considered to be neutral with regard to their influence on serum lipids and lipoproteins. However, studies have suggested that MUFAs may also have favorable effects on blood lipid concentrations. Two meta-analysis reported that a high MUFA-diet reduces fasting plasma triacylglycerol levels, as well as the susceptibility of LDL particles to oxidation; even no changes were noted in concentrations of HDL or LDL cholesterol (7,8). Zheng et al. have suggested that MUFA intake activates synthetic and rapid catabolic pathways for triglyceride-rich lipoprotein metabolism that involve apolipoprotein E and apolipoprotein C-III, and suppresses the metabolism of more slowly metabolized VLDLs and doubles the direct clearance of triglyceride-rich lipoprotein from the circulation (9).
Studies have shown that the MUFA-diet increases insulin secretion (7,10). However, few studies have attempted to investigate the mechanisms by which dietary MUFAs mediate these benefits, and a hypothesis would explain this effect including the incretin hormone pathway. Paniagua et al. (10) and Rocca et al. (11) have found that MUFA increases secretion of glucagon-like peptide-1 (GLP-1). According to López et al. (12), MUFA may modulate the postprandial hyperactivity of beta-cells through GLP-1 and glucose-dependent insulinotropic polypeptide (gastric inhibitory polypeptide [GIP]). GLP-1 has an antidiabetic action through its ability to stimulate insulin secretion, inhibit beta cell apoptosis, inhibit glucagon secretion, and delay gastric emptying and induce satiety. GIP also promotes energy storage via direct actions on adipose tissue. Therefore, stimulating GIP and GLP-1 secretion results in a positive stimulation of beta-cells to increase insulin secretion (11).
MUFA may also be an agonist for PPAR, because MUFAs inhibit acyl-CoA oxidase, which is involved in beta-oxidation, to indirectly increase accumulation of acyl-CoA leading to PPAR activation (13).
Since the precise mechanisms underlying the beneficial effects of these fatty acids are not yet fully understood, and they are natural PPAR ligands, we investigated the influence of unsaturated fatty acids intake on anthropometric and biochemical variables in obese woman, carriers of the wild-type homozygous genotype in the PPARγ gene (Pro12Pro).
Methods
Casuistry
This study was conducted in the Institute of Nutrition Josué de Castro at the Federal University of Rio de Janeiro (Brazil).
Volunteers were recruited through poster advertisements at the Clementino Fraga Filho University Hospital, Brazil (between March 2006 and October 2007).
The sample size and the selection of women were for convenience (14). All participants signed an informed consent, and the study was approved by the Ethical Committee (Institutional Review Board, protocol 116/05).
The inclusion criteria considered were: adult women with a family history of obesity, lack of menopause, and BMI greater than 35 kg/m2 (15).
Exclusion criteria were smoking, alcoholism, cardiovascular diseases, chronic kidney disease, diabetes mellitus and/or other chronic diseases, infectious diseases, pregnancy, use of antibiotics or anti-inflammatory drugs, antidiabetic medications, lipid-lowering drugs, diuretics, antidepressants, antihypertensive, and drugs supplements and/or herbal remedies for weight loss, dieting for weight loss in the last four weeks, or weight loss greater than 3 kg in the last month.
Study Desing
This is a controlled randomized clinical-trial. All volunteers were assessed at baseline and after forty-five days of intervention.
The participant arrived at the Laboratory of Clinical Analysis of the Pharmacy College at 7 h a.m. after twelve hours overnight fast. Upon arrival, venous blood glucose samples were collected and the anthropometric assessment was performed immediately after.
Participants were allocated into two groups to receive a diet rich in PUFA (PUFA-diet) or a diet rich in MUFA (MUFA-diet).
Fortnightly, they received individual face-to-face consultation sessions which included advices on food purchase and selection, portion sizes, and cooking methods. In these consultations, anthropometry was assessed and 24-hour recalls were performed to verify adherence to the diet.
Dietetic intervention
All participants received an individualized diet based on the total energy expenditure (estimated according to FAO/WHO [16]) with an energy deficit of 500-1.000 kcal/day achieved through reductions in total energy intake (17).
Energy from macronutrients was similar in both groups (dietary energy content of 50-60% carbohydrates, 15-20% of protein, 30% of total fat), and both diets have less than 10% of saturated fatty acids (SFA), based on current recommendations (16).
The PUFA-diet consisted of 15% of PUFA and 10% of MUFA, and the MUFA-diet consisted of 10% of PUFA and 15% of MUFA.
The analysis of the chemical composition of the diets was conducted in Food Processor program version 12 (Esha Research, Salem, USA, 1984).
Biochemical Measurements
Total cholesterol, HDL-cholesterol and triglycerides were measured using the commercial kits (Cholesterol Liquiform, Labtest Diagnostica S.A., Brazil; HDL Cholesterol, Labtest Diagnostica S.A., Brazil; and triglycerides Liquiform, Labtest Diagnostica S.A., Brazil).
LDL-cholesterol concentrations were determined using the Friedewald equation (18).
The determination of plasma glucose was performed using the commercial kit GLUCOSE PAP Liquiform (Labtest Diagnostica S.A., Brazil).
Serum insulin was analyzed using the commercial kit COAT-A-Count® (Diagnostic Products Corporation®, USA). Hyperinsulinemia was considered in volunteers with fasting insulin > 9 μU/Ml(19).
Insulin resistance (IR) was estimated by calculating homeostasis model assessment (HOMA-IR). IR values were considered as HOMA-IR ≥ 2.71 (20).
Anthoropometry assessment
BMI was calculated as body weight in kilograms divided by the square of height in meters (15).
Waist circumference (WC) was determined by the average of two measurements obtained at the midpoint between the lower rib margin and the iliac crest after a normal expiration (21).
Hip circumference was determined around the widest portion of the hip, and the waist-hip ratio was calculated as well (21).
Genotyping Pparγ2
Molecular analyses were performed in the Laboratory of Molecular Biology of Cancer, at the Federal University of Rio de Janeiro.
Genomic DNA was extracted from samples of whole blood using a commercial kit (MasterPureTM Genomic DNA Purification Kit, Epicentre®, Biotechnologies) and stored at -20 oC until the subsequent step.
Determination of the Pro12Pro genotype was performed using the polymerase chain reaction-restriction fragment-length polymorphism (PCR-RFLP) method, according to the sequences available in the Gen Bank DNA AB005520, and according previous described for us (22).
The sequences of PCR primers were: 5'-GCC AAT TCA AGC CCA GTC-3 'and 5'-GCC ATG TTT GCA GAC AGT GTA TCA GTG AAG GAA TCG CTT TCC G- 3'. The cycling conditions were as follows: an initial denaturation at 95 oC for 5 minutes, followed by 35 cycles of denaturing at 95 oC for 30 seconds, annealing at 59 oC for 30 seconds and extension at 72 oC for 30 seconds. The final extension was continued at 72 oC for 10 minutes and cooling to 4oC. The generated fragment was 267 bp (base pairs).
After enzymatic digestion of the PCR products (60 oC for 180 minutes) by Bst UI restriction endonuclease (New England Biolabs, Inc.), fragments of 267 bp were generated, indicating the presence of wild type homozygous genotype (Pro12Pro).
Dietary Intake Assessment
Volunteers filled in a 3-day food record (2 weekdays and 1 weekend day). During those three days, all foods and drinks consumed had to be documented to allow quantitative estimation of dietary intake. The 3-day energy and nutrient intakes were averaged to obtain a mean daily energy and nutrient intake for each volunteer.
Volunteers were followed fortnightly when 24-hour recalls were performed to verify adherence to the diet.
The individual food records of each volunteer were carefully checked, and data were then entered into the nutritional software (Food Processor software version 12, Esha Research, Salem, USA, 1984), after the adjustment for the typical Brazilian diet.
Statistical Analysis
Statistical analyses were performed in SPSS software (version 17.0; SPSS Inc, Chicago, IL) with 5% significance level.
To check the distribution of continuous variables (clinical, anthropometric and biochemical) the test of Kolmogorov-Smirnov was performed (1: age; 2: body weight, BMI and WC; 3: serum insulin, plasma glucose, triglycerides, total cholesterol and fractions [HDL, LDL and VLDL] and values of HOMA-IR and QUICK).
For the comparison between the means of the groups, the basic statistics of location (mean) and dispersion (standard deviation) were calculated.
Continuous variables presented normal distribution and the parametric Student t test for the comparison between groups was used. When the variance was less than 4, we used the Student t test for equal variances; otherwise, we applied the Student t test for different variances.
Results
Among women recruited, only one had the genotype variant (Ala12Ala), and she was therefore excluded from the study. All others eighteen volunteers presenting the genotype Pro12Ala were included in the study.
Ten volunteers (55.5%) used the MUFA-diet while eight (44.4%) used the PUFA-diet.
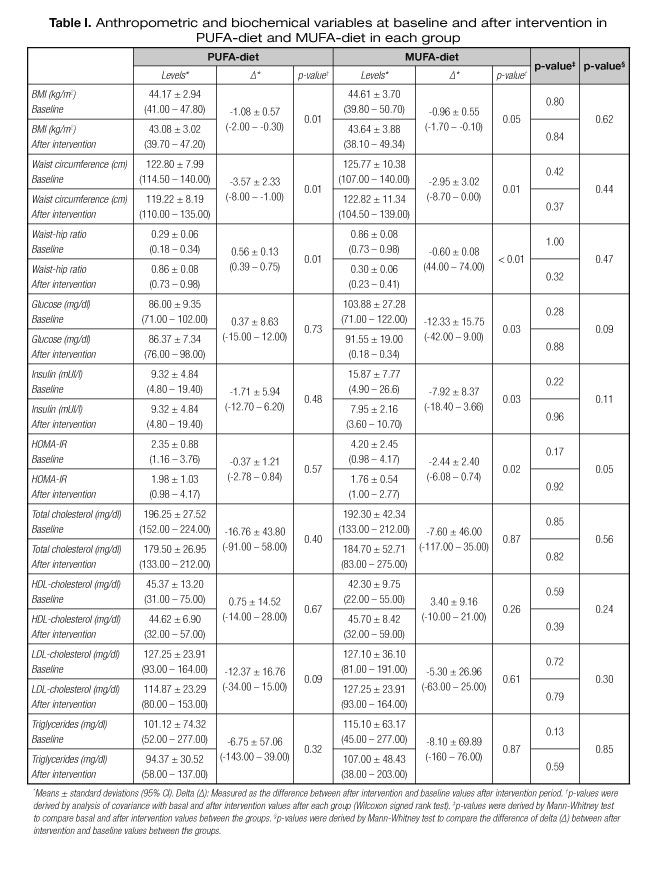
The characteristics of each group are presented in table I. They presented a mean age of 36.7 ± 6.08 (IC: 23-46), and a mean BMI of 44 ± 3.31 kg/m2 (IC: 39.8-50.70). Anthropometric and biochemical characteristics were similar between groups at baseline (p > 0.05).
Characteristics of groups after intervention
The characteristics of groups after intervention are also presented in table I.
Waist-hip ratio increased in PUFA-diet (0.56 ± 0.13; p = 0.01) and decreased in MUFA-diet (-0.60 ± 0.08; p < 0.01), and WC decreased in both groups (PUFA-diet: -3.57 ± 2.33 cm; MUFA-diet: -2.95 ± 3.02 cm) (p = 0.01). BMI decreased only in PUFA-diet (-1.08 ± 0.57 kg/m2; p = 0.01), and only MUFA-diet decreased fasting glucose (-12.33 ± 15.75 mg/dl; p = 0.03), insulin (-7.92 ± 8.37 mUI/l; p = 0.03) and HOMA-IR (-2.44 ± 2.40; p = 0.02).
However, the variation (delta) after intervention and baseline values between groups showed that the anthropometric and biochemical variables did not differ between PUFA and MUFA-diets (p > 0.05).
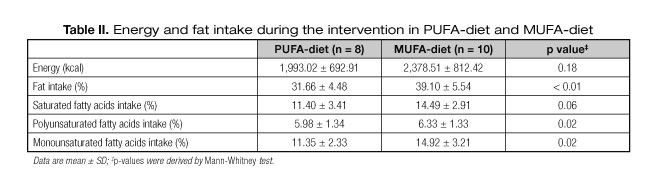
As shown in table II, there was no significant difference between groups for energy intake (p > 0.05) as calculated from 3-day food records. However, total fat was higher in MUFA-diet compared to PUFA-diet (p < 0.01). As we wanted, PUFA intake was higher in the PUFA-diet group (p = 0.02), while the group with MUFA-diet presented a higher MUFA intake (p = 0.02). Both groups intake more than 10% of SFA, however, there was no difference between groups. The other nutrients did not differ between groups (p > 0.05).
PUFA intake was not associated with any of the anthropometric or laboratory variables (p > 0.05). However, MUFA intake showed a negative association with the HOMA-IR (r = -0.52; p = 0.03) in both groups, and regression analysis showed an association between the HOMA-IR and MUFA intake only in MUFA-diet (r = 0.71; p = 0.02).
Discussion
In the present study, no differences were found in anthropometric and biochemical variables comparing PUFA- and MUFA-diets. However, the intragroup evaluation has shown that BMI decreased only in PUFA-diet, and fasting glucose, insulin and HOMA-IR decreased only in MUFA-diet. The methodological care in this study is an advantage, since other studies evaluating the effect of unsaturated fats in weight loss and lipid and glucose profile do not standardize the studied population considering the presence of genetic variants, particularly of nuclear transcription factors.
We only recruit women carriers of the homozygous genotype (Pro12Pro) because the presence of genetic variant may alter the lipidic and glicidic metabolism (23).
Studies have supported the fact that MUFAs and PUFAs may act as ligands of PPARγ2, increasing GLUT4 transcription and improving insulin resistance (24,25). Our results demonstrated that MUFA intake is more effective than PUFA intake to improve HOMA-IR values.
Studies have shown that MUFA intake may increase the response of pancreatic beta-cells to improve insulin sensitivity, increase incretins production (like GLP-1) and reduce insulin clearance (10,11). Thus, guidelines affirm that high MUFA-diet improves HOMA-IR in insulin-resistant subjects (26).
Previous studies identified no difference in insulin sensitivity between diets rich in SFA versus MUFA or PUFA versus MUFA, or all three (27,28). However, other studies have showed conclusions similar to our results (29,30). Thus, based on these data, we suggest a preference for MUFA over PUFA for improved insulin sensitivity.
Our results showed that PUFA-diet increased and MUFA-diet reduced the waist-hip ratio, and both unsaturated fatty acids have reduced the waist circumference. Therefore, improvement in insulin sensitivity was not associated with reduction of waist circumference, but with the possible favorable effect of the MUFA-diet on endothelial function.
Previous studies demonstrated that a high dietary SFA or n-6 fatty acids (PUFA) are significant independent predictors of fasting hyperinsulinemia (27,28). In contrast, the favorable effect of the MUFA-diet on endothelial function might be attributed to the inhibition of the expression of leukocyte adhesion molecules (7-9). Therefore, we suggest that the specific dietary fat may influence body fat distribution and insulin sensibility without affecting total body weight.
In addition, we found a BMI reduction in response to caloric restriction, independently of unsaturated fatty acids diets. This is consistent with Garaulet et al. study, which reported a lesser resistance to weight loss in individuals with the Pro12 carriers (31).
Even though no significant differences between groups were found, we observed that the PUFA-diet led to a greater decrease of body weight compared with MUFA-diet. We know that PUFA serves as a precursor for prostaglandins, which play a critical role in β-oxidation and the expression of PPAR-dependent and PPAR-independent pathways that are involved in adipogenesis and lipid storage (32), but the links between the coordination of other metabolic gene expression remain unclear. Therefore, regardless of the proportion of dietary fat, the group with PUFA-diet consumed less total dietary fat than the group with MUFA-diet (31.66 ± 4.48% and 39.10 ± 5.54%, respectively; p < 0.01).
A reduction of dietary fat is one of the most practical ways to reduce energy density, and dietary fats have less effect on satiety, promote energy overconsumption (33) and have a lower oxidative priority compared to proteins and carbohydrates (34).
We are aware of certain limitations of the study. First, the sample was small and selected for convenience (14). Therefore, these results should be interpreted with caution, and the analysis of the effect of PUFA and MUFA-diets in PPARγ2 Pro12Pro carriers needs to be replicated in additional large-scale studies. Second, participants may not have recalled everything that they ate and were unable to estimate portion sizes accurately (35). This fact may underestimate or overestimate the total fatty acids intakes of groups.
Conclusions
To conclude, current data show that MUFA-diet is more efficient than PUFA-diet to reduce the insulin resistance in obese women with Pro12Pro genotype in PPARγ2, despite the high total fat and SFA content in MUFA-diet.
Acknowledgements
We would like to thank volunteers who participated in the study, Sofia Kimi Uehara (Ph. D. Nutrition Sciences at Federal University of Rio de Janeiro), Dr. Franklin D. Rumjanek, Nivea Amoedo and other employees of the Laboratory of Molecular Biology of Cancer, at the Federal University of Rio de Janeiro; Dr. Maria de Fátima Santos de Oliveira and other professionals from the Association of Parents and Friends of Exceptional Children-Tijuca, Rio de Janeiro; and Dr. Marcos Fleury, from the Laboratory of Clinical Analyses of the Pharmacy College of the Federal University of Rio de Janeiro, and other laboratory employees.
References
1. Kopelman PG. Obesity as a medical problem. Nature 2000;404(6778):635-43. [ Links ]
2. Kopelman P. Health risks associated with overweight and obesity. Obes Rev 2007;8:13-7. [ Links ]
3. Deeb SS, Fajas L, Nemoto M, Pihlajamäki J, Mykkänen L, Kuusisto J, et al. A Pro12Ala substitution in PPARγ2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 1998;20(3):284-7. [ Links ]
4. Yen C-J, Beamer BA, Negri C, Silver K, Brown KA, Yarnall DP, et al. Molecular scanning of the human peroxisome proliferator activated receptor γ (hPPARγ) gene in diabetic Caucasians: Identification of a Pro12Ala PPARγ2 missense mutation. Biochem Biophys Res Commun 1997;241(2):270-4. [ Links ]
5. Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Progress in Lipid Res 2008;47(2):147-55. [ Links ]
6. Reginato MJ, Krakow SL, Bailey ST, Lazar MA. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma. J Biol Chem 1998;273(4):1855-8. [ Links ]
7. Garg A. High-monounsaturated-fat diets for patients with diabetes mellitus: A meta-analysis. Am J Clin Nutr 1998;67(3):577S-82S. [ Links ]
8. Cao Y, Mauger DT, Pelkman CL, Zhao G, Townsend SM, Kris-Etherton PM. Effects of moderate (MF) versus lower fat (LF) diets on lipids and lipoproteins: A meta-analysis of clinical trials in subjects with and without diabetes. J Clin Lipidol 2009;3(1):19-32. [ Links ]
9. Zheng C, Khoo C, Furtado J, Ikewaki K, Sacks FM. Dietary monounsaturated fat activates metabolic pathways for triglyceride-rich lipoproteins that involve apolipoproteins E and C-III. Am J Clin Nutr 2008;88(2):272-81. [ Links ]
10. Paniagua JA, De la Sacristana AG, Sánchez E, Romero I, Vidal-Puig A, Berral FJ, et al. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J Am Coll Nutr 2007;26(5):434-44. [ Links ]
11. Rocca AS, LaGreca J, Kalitsky J, Brubaker PL. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinol 2001;142(3):1148-55. [ Links ]
12. López S, Bermúdez B, Pacheco YM, Villar J, Abia R, Muriana FJ. Distinctive postprandial modulation of β cell function and insulin sensitivity by dietary fats: Monounsaturated compared with saturated fatty acids. Am J Clin Nutr 2008;88(3):638-44. [ Links ]
13. Yokoi H, Mizukami H, Nagatsu A, Tanabe H, Inoue M. Hydroxy monounsaturated fatty acids as agonists for peroxisome proliferator-activated receptors. Biol Pharm Bull 2010;33(5):854-61. [ Links ]
14. Lwanga SK, Lemeshow S. Sample size determination in health studies: A practical manual. Geneva: WHO; 1991. [ Links ]
15. World Health Organization. Physical status, the use and interpretation of anthropometry: Report of a WHO expert committee. World Health Organization, Geneva; 1995. [ Links ]
16. Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 2002;102(11):1621-30. [ Links ]
17. Wishnofsky M. Caloric equivalents of gained or lost weight. Am J Clin Nutr 1958;6(5):542-6. [ Links ]
18. Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499. [ Links ]
19. Sánchez-Margalet Vc, Valle M, Ruz FJ, Gascón F, Mateo Jn, Goberna R. Elevated plasma total homocysteine levels in hyperinsulinemic obese subjects. J Nutr Biochem 2002;13(2):75-9. [ Links ]
20. Geloneze B, Repetto E, Geloneze S, Tambascia M, Ermetice M. The threshold value for insulin resistance (HOMA-IR) in an admixtured population. Diabetes Res Clin Pract 2006;72(2):219-20. [ Links ]
21. World Health Organization. Waist Circumference and Waist-Hip ratio: Report of a WHO Expert Consultation. 2008. Geneva: WHO; 2008. p. 8-11. [ Links ]
22. Kaippert VC, Uehara SK, D'Andrea CL, Nogueira J, Do Lago MF, Dos Santos Lopes MCO, et al. Influence of the body mass and visceral adiposity on glucose metabolism in obese women with Pro12Pro genotype in PPARgamma2 gene. Nutr Hosp 2013;28(3):694-700. [ Links ]
23. Rosado EL, Bressan J, Martinez JA, Marques-Lopes I. Interactions of the PPARgamma2 polymorphism with fat intake affecting energy metabolism and nutritional outcomes in obese women. Ann Nutr Metab 2010;57(3-4):242-50. [ Links ]
24. Barroso I, Gurnell M, Crowley VEF, Agostini M, Schwabe JW, Soos MA, et al. Dominant negative mutations in human PPAR(gamma) associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999;402(6764):880-3. [ Links ]
25. Nugent C, Prins JB, Whitehead JP, Wentworth JM, Chatterjee VKK, O'Rahilly S. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor γ. J Biol Chem 2001;276(12):9149-57. [ Links ]
26. American Diabetes Association Nutrition. Therapy recommendations for the management of adults with diabetes. Diabetes Care 2014;37(1):S120-S43. [ Links ]
27. Lovejoy JC, Smith SR, Champagne CM, Most MM, Lefevre M, DeLany JP, et al. Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes Care 2002;25(8):1283-8. [ Links ]
28. Louheranta AM, Turpeinen AK, Vidgren HM, Schwab US, Uusitupa MIJ. A high-trans fatty acid diet and insulin sensitivity in young healthy women. Metab Clin Exp 1999;48(7):870-5. [ Links ]
29. Kien CL, Bunn JY, Poynter ME, Stevens R, Bain J, Ikayeva O, et al. A lipidomics analysis of the relationship between dietary fatty acid composition and insulin sensitivity in young adults. Diabetes 2013;62(4):1054-63. [ Links ]
30. Westerterp KR, Smeets A, Lejeune MP, Wouters-Adriaens MP, Westerterp-Plantenga MS. Dietary fat oxidation as a function of body fat. Am J Clin Nutr 2008;87(1):132-5. [ Links ]
31. Garaulet M, Smith CE, Hernández-González T, Lee YC, Ordovás JM. PPARγ Pro12Ala interacts with fat intake for obesity and weight loss in a behavioural treatment based on the Mediterranean diet. Mol Nutr Food Res 2011;55(12):1771. [ Links ]
32. Al-Hasani H, Joost H. Nutrition-/diet-induced changes in gene expression in white adipose tissue. Best Pract Res Clin Endocrinol Metab 2005;19(4):589. [ Links ]
33. Blundell JE, MacDiarmid JI. Fat as a risk factor for overconsumption: Satiation, satiety, and patterns of eating. J Am Diet Assoc 1997;97(7):S63-9. [ Links ]
34. Astrup A, Buemann B, Western P, Toubro S, Raben A, Christensen NJ. Obesity as an adaptation to a high-fat diet: Evidence from a cross-sectional study. Am J Clin Nutr 1994;59(2):350-5. [ Links ]
35. Thompson FE, Subar AF, Loria CM, Reedy JL, Baranowski T. Need for technological innovation in dietary assessment. J Acad Nutr Diet 2010;110(1):48-51. [ Links ]
![]() Correspondence:
Correspondence:
Débora Lopes Souto.
Department of Nutrition and Dietetics.
Federal University of Rio de Janeiro,
Institute of Nutrition Josué de Castro.
373 Carlos Chagas Filho
Av. Health Sciences Center. Sector J, 2nd floor.
Ilha do Fundão.
21941-590 Rio de Janeiro. Brazil
e-mail: deboralopessouto@gmail.com
Received: 15/09/2015
Accepted: 02/11/2015