Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.33 no.2 Madrid mar./abr. 2016
https://dx.doi.org/10.20960/nh.525
The effect of orlistat on postprandial hypertriglyceridemia by oral fat loading test. A systematic review
Efecto de orlistat sobre la hipertrigliceridemia posprandial valorada mediante el test de sobrecarga oral de grasas. Revisión sistemática
Ana Rodríguez-Valle1, María Ángeles Navarro Ferrando2, Diana Boj Carceller3, Mar González-Cantalejo4, Jesús Fernando Escanero Marcén2 and Alejandro Sanz-Paris3
1 Department of Clinical Biochemistry. Hospital Universitario Miguel Servet de Zaragoza. Zaragoza, Spain.
2 Universidad de Zaragoza. CIBER-OBN, IIS. Zaragoza, Spain.
3 Unit of Dietetics and Nutrition and
4 Biblioteca Médica. Hospital Universitario Miguel Servet. Zaragoza, Spain
ABSTRACT
Orlistat induces weight loss by blocking hydrolysis of triglyceride in the intestine, and has thereby been associated with favorable changes in postprandial triglycerides (ppTGL). Some epidemiological studies have identified ppTGL concentrations as a significant risk factor for cardiovascular disease. Oral fat loading test (OFLT) has been used for screening of elevated levels of ppTGL. The objective of the present systematic review is to present available data on the effects of orlistat on OFLT.
We found 11 studies, seven of which studied the effect of a single dose of orlistat on OFLT in three healthy volunteers, one with obesity, two with type-2 diabetes and one with hyperlipidemic patients. The other four studied the effect of orlistat on OFLT, but after a previous period of time with daily treatment with orlistat: 1 healthy volunteer, 2 obese volunteers, and one patient with hyperlipidemia.
Our systematic review suggests that orlistat can help to reduce postprandial hypertriglyceridemia in obese, dyslipemic and type-2 diabetic patients. Regarding free fatty acids, they could be reduced but not all the authors have found the same results. In relation to type-2 diabetic patients, we have found three studies with conflicting results on the immediate effect of orlistat on the postprandial GLP-1 response.
In conclusion, orlistat can help to reduce postprandial plasmatic TGL, especially in patients with postprandial hypertriglyceridemia related to obesity, dyslipidemia or type-2 diabetes.
Key words: Orlistat. Postprandial triglycerides.
RESUMEN
Orlistat induce la pérdida de peso mediante el bloqueo de la hidrólisis de triglicéridos en el intestino, por lo que también se asocia con cambios favorables en los triglicéridos posprandiales (PHTGL). Algunos estudios epidemiológicos han identificado concentraciones PHTGL como un importante factor de riesgo para la enfermedad cardiovascular. El test de sobrecarga oral de grasa (TSOG) se ha utilizado para la detección de niveles elevados de PHTGL. El objetivo de la presente revisión sistemática es presentar los datos disponibles sobre los efectos de orlistat en TSOG.
Encontramos 11 estudios, de los cuales 7 estudian el efecto de una sola dosis de orlistat en el TSOG: 3 con voluntarios sanos, 1 con obesidad, 2 con diabetes de tipo 2 y 1 con pacientes hiperlipidémicos. Los otros 4 estudian también el efecto de orlistat en el TSOG, pero después de un periodo de tiempo previo con un tratamiento diario con orlistat: 1 con voluntarios sanos, 2 con obesidad y 1 con un paciente con hiperlipidemia.
Nuestra revisión sistemática sugiere que orlistat puede ayudar a reducir la hipertrigliceridemia posprandial en pacientes obesos, dislipémicos y con diabetes de tipo 2. Respecto a los ácidos grasos libres plasmáticos, también los podría reducir, pero no todos los autores han encontrado los mismos resultados. En pacientes diabéticos de tipo 2 se han encontrado tres estudios con resultados contradictorios sobre el efecto inmediato de orlistat en la respuesta postprandial de GLP-1.
En conclusión, orlistat puede ayudar a reducir los TGL plasmáticos posprandiales, especialmente en pacientes con hipertrigliceridemia posprandial relacionada con obesidad, dislipemia y diabetes de tipo 2.
Palabras clave: Orlistat. Triglicéridos posprandiales.
Introduction
Humans consume regular meals during the day; therefore, we are in a dynamic state of continuous changes in postprandial lipid and lipoprotein metabolism. Postprandial lipemia is a normal physiological phenomenon that usually lasts from 6 to 8 hours after ingestion of a fatty meal. However, postprandial hypertriglyceridemia (PHTGL) is an exaggerated magnitude and duration of triglyceride (TGL) response after a fatty meal, which results in the accumulation of TGL and their remnants in the circulation.
The causes of PHTGL include: decreased hepatic receptor activity, defect in the ligand for these receptors, impaired lipolysis by lipoprotein lipase (LPL) and Apo C (Apolipoprotein C) dysregulation by acting as an inhibitor of LPL and TGL remnant uptake by hepatic lipoprotein receptors (1).
The atherogenicity of TGL relates to the smaller remnant particles that readily infiltrate the subendothelial space of the arterial wall. These TGL remnants are phagocytized by arterial wall macrophages, which then transform into foam cells, impair endothelial function, active monocytes and inflammatory signaling pathways, and also have a direct effect on thrombogenicity (2,3).
Some epidemiological studies have identified non-fasting TGL concentrations as a significant risk factor for cardiovascular disease. Copenhagen City Heart study (4), Women's Health study (5) and the Norwegian Counties study (6), all found highly significant associations between cardiovascular events with increases in non-fasting TGL.
Furthermore, the findings in the 31st year follow-up of Copenhagen City Heart study (4) were in agreement with its previous data, with non-fasting TGL being similarly associated with an increased risk of myocardial infarction, ischemic heart disease (7), and ischemic stroke (8). In addition, there is a meta-analysis of 101 studies suggesting the causal role of plasma TGL in coronary disease (9).
The role of TGL has been emphasized by recent major guidelines. A therapeutic fasting target level of triglyceride of less than 150 mg/dL is recommended in higher-risk patients, especially those with diabetes and metabolic syndrome (10,11).
Non-fasting TGL cutoff levels of ≥ 180 mg/dL and > 220 mg/dl after the Oral Fat Loading Test (OFLT) have been used for screening and management of elevated TGL levels (12).
Dietary and lifestyle modifications remain the cornerstone of therapeutical management, whose function is to lower plasmatic TGL, and this is the first-step approach. Subsequent steps are pharmacotherapies including fibrates, niacin (currently not available in Spain), and n-3 fatty acids.
New therapies, such as dual peroxisome-proliferator-activated receptor α/δ agonists, diacylglycerol, inhibitors of diacylglycerol acyltransferases-1, microsomal triglyceride transfer protein, antisense oligonucleotides for apoB-100 and apoC-III, and incretin-based therapies could be also employed to optimize the treatment.
Orlistat is a drug approved in the year 1999 for long-term weight loss in adults. Orlistat induces weight loss by blocking the hydrolysis of triglyceride in the stomach and small intestine, thereby, inhibiting absorption of approximately 30% of dietary fat.
Anti-obesity drugs help to reduce body weight, thus, contributing to an improvement in cardiovascular risk factors. However, the direct effect of anti-obesity drugs on cardiovascular risk factors is unclear (13). Orlistat not only induced weight loss, but it was also associated with favorable changes in lipids, fasting glucose, and blood pressure. The Orlistat Swedish Multi-morbidity Study showed greater improvement in coronary risk factors with orlistat than with diet changes alone in obese patients with hypertension, hypercholesterolemia, and/or type 2 diabetes (14). These results have been observed in other studies (15). More so, the metabolic effects of orlistat are not only limited to cardiovascular risk factors, but also to a lower incidence of type 2 diabetes (16).
Orlistat may represent a pharmacological approach to cardiovascular risk modulation in overweight/obese patients, although no agency has admitted that indication. The objective of the ongoing systematic review is to present available data on the effects of orlistat on OFLT.
Methods
SEARCH SRATEGY
A literature search has been made in the following medical databases through June 2015: Medline (PubMed), Embase, Cochrane Library and Trip Database. National and international agencies like the CRD (Centre for Reviews and Dissemination), NICE (National Institute for Health and Care Excellence), HEN (Health Evidence Network) , IQWIG (Institute for Quality and Efficiency in Health Care), INAHTA (International Network of Agencies for Health Technology Assessment), EuroScan, NHSC (NIHR Horizon Scanning Centre), AHRQ (Agency for Healthcare Research and Quality), CEDIT (Comité d'évaluation des technologies de santé), CADTH (Canadian Agency for Drugs and Technologies in Health), UKMI (United Kingdom Medicines Information, NHS), EMEA (European Medicines Agency), AEMPS (Spanish Agency for Medicines and Health Products), AETS-ISCIII (Agency for Health Technology Assessment of the Institute Carlos III, Spain), OSTEBA (Service Health Technology Assessment of the Basque Country), AETSA (Agency for Health Technology Assessment Andalusia) were also revised.
The literature search in medical databases followed these steps:
1. "Hyperlipidemias" [mesh] and "Postprandial period" [mesh].
2. Postprandial lipemia [title/abstract] OR postprandial lipid [title/abstract] or postprandial hypertriglyceridemia [title/abstract] OR postprandial triglyceride [title/abstract] OR postprandial triglycerides [title/abstract] OR postprandial lipaemia [title/abstract] OR postprandial hyperlipidemia [title/abstract] OR oral lipid tolerance test [title/abstract] OR postprandial [title/abstract].
3. #1 OR #2.
4. "Orlistat" [supplementary concept].
5. Xenical [title/abstract] OR orlistat [title/abstract].
6. #4 OR #5.
7. #3 AND #6.
A filter for studies in humans was also used. The search was restricted to articles in English and Spanish languages. The search was refined by eliminating duplicate documents.
The retrieved studies were manually screened to assess their appropriateness for inclusion. Bibliographies of all identified trials and review articles were reviewed to look for additional studies of interest.
An investigator reviewed all the citations retrieved from the electronic search in order to identify relevant articles for this review. Other investigators subsequently reviewed the selection, to determine eligibility.
INCLUSION AND EXCLUSION CRITERIA
Inclusion criteria: Studies with healthy, cardiovascular or metabolic disease participants, regardless of their body mass index (BMI), their age (> 18 years), with no chronic medication, who underwent an OFLT were included.
Exclusion criteria: Reports, editorials and review articles were excluded.
DATA EXTRACTION AND QUALITY EVALUATION
All articles provided measures for blood determinations, including mean and standard deviation (SD) or standard error of the mean (SEM), at least at baseline and at one other postprandial time point.
The following data were abstracted in tables onto the standardized case report forms: authors; year of publication; number of patients, sex, age, BMI and characteristics of the treatment and control groups (healthy, obese, hyperlipidemia, metabolic syndrome or diabetes); study goals; methods of randomization and blinding; duration and characteristics of treatment; administered fat load test; outcomes; and adverse event data.
A validated 3-item scale was used to assess the overall reporting quality of the selected studies for inclusion in the present review (17). This scale provides scoring for randomization (0-2 points), double-blinding (0-2 points), and account for withdrawals (1 point). Scores ranged between 0 and 5, and scores ≥ 3 indicate a high quality study.
Results
A total of 78 citations were identified by the systematic literature search (66 from medical databases and 12 from national and international agencies), 49 of which were deemed potentially eligible. 38 of the 49 articles were excluded because they did not meet the inclusion criteria. Thus, a total of 11 trials were eligible for analysis (18-28), only 7 of which were double-blind randomized controlled trials (RCT) (18,19,22-26) (Fig. 1).
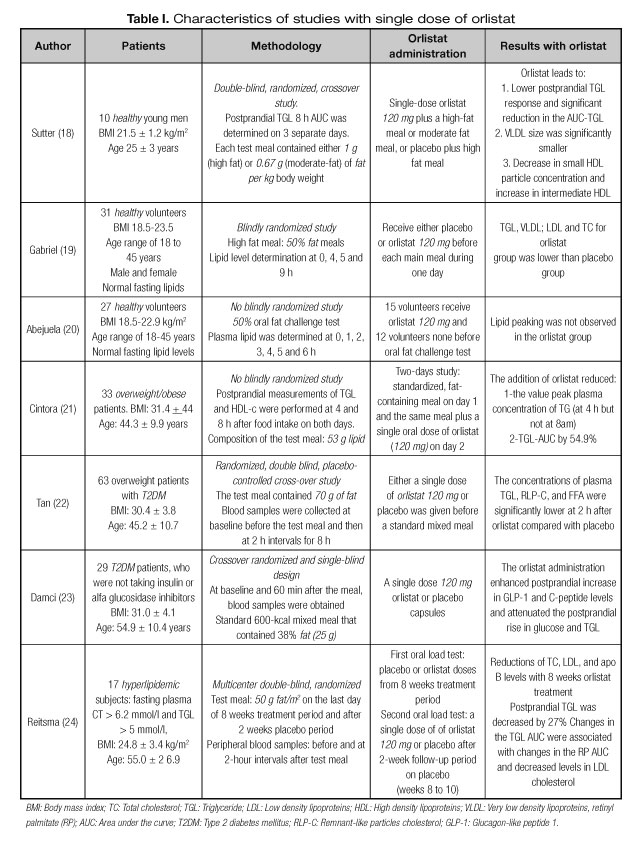
A total of 7 papers (18-24) studied the effect of a single dose of orlistat on OFLT (Table I), 3 of which were carried out with healthy volunteers (18-20), 1 with obese patients (21), 2 with type 2 diabetes patients (22,23), and 1 with hyperlipidemic patients (24).
Of the total 11 included studies, only 4 studied the effect of orlistat on postprandial lipemia, after a period of time with a daily treatment using orlistat (24-28) (Table II). Among these, 1 was carried out with healthy volunteers (25), 2 with obese (26,27), and 1 with hyperlipidemic patients (24).
Only one study conducted an OFLT after 8 weeks of orlistat treatment and a second OFLT after two weeks of placebo (24), so it is included in all tables.
STUDY CHARACTERISTICS
The characteristics of the included studies are listed in tables I and II, and summarized in tables III and IV.
There are two studies that are not randomized or blinded (20,21), but they were included due to the number of patients. The dose of fat used in the OFLT was variable, with about 28 grams (25) to 60 grams per square meter of body surface (27). In many studies, the exact dose of fat used is not clearly defined. In this regard, studies are not comparable. However, the dose of Orlisat used was always 120 mg, as a single dose or in short treatment (before breakfast, lunch and dinner). In most studies, TGL area under the curve (AUC) was calculated. Meta-analysis was not considered because test meals, duration of observation in the postprandial state, and the type of patients differ within the study.
HEALTHY VOLUNTEERS
We found that 3 studies were done with a single dose of orlistat in healthy volunteers (18-20), which make up 68 individuals in all.
The response to the OFLT was homogeneous in all patients with a reduced postprandial triglyceride AUC compared to placebo. In addition, a significant reduction of total LDL cholesterol levels (19,20), HDL cholesterol (18,20) and VLDL (18,19) was observed.
Regarding the response of postprandial free fatty acids (FFA), glucose and insulin, only one study explored this (18), with no significant results.
After 10 days of treatment with orlistat, Shepard et al. (25), in an article of high methodological quality, found no significant difference regarding the postprandial response with orlistat compared to placebo in any of the previously mentioned parameters.
NORMOLIPEMIC OBESE POST MENOPAUSE FEMALE
We have found only two articles with normolipemic obese post menopause female.
Cintora et al. (21) conducted an OFLT with orlistat and observed an attenuation of the AUC and peak of TGL at 4 hours.
Later, Di Somma et al. (26) performed an OFLT after 10 days of daily treatment with orlistat. The result showed lower postprandial response in the treatment group in total cholesterol, LDL cholesterol, HDL cholesterol, FFA and insulin, but not in TGL.
DISLIPEMIA
We have found three papers with different types of dyslipidemia: obese with only PPL (27), obese with metabolic syndrome (28) and obese with fasting hyperliperlipidemia (24). In all three studies, the authors observed a decreased response of TGL after the OFLT with orlistat. The reduction of TGL was significantly correlated with decreased fasting TGL, insulin and HOMA after 12 weeks or after 10 days of orlistat treatment.
TYPE 2 DIABETES MELLITUS
In two studies (22,23) TGL lower postprandial response is observed with administration of orlistat. Postprandial FFA response in a paper is lower (22) whereas in the other it is not significant (23). Tan et al. (22) describe lower peak and AUC of remnant like particles cholesterol. Damci et al. (23) showed attenuated glucose response with enhanced C-peptide and GLP-1 response.
Discussion
Orlistat is a synthetic hydrogenated derivative of lipstatin that partially inhibits gastric lipase, pancreatic lipase and carboxyl ester lipase enzymes. It reduces the absorption of ingested fat by 30%, increasing its excretion in the feces.
The European Medicines Agency (29) and the American FDA (30) approved its use only as a prescription agent for long-term weight loss in adults with a BMI > 30 kg/m2 or a BMI > 27 kg/m2, with comorbidities and in conjunction with a reduced-calorie diet.
Therefore, orlistat could improve lipid metabolism, ameliorating postprandial TGL increase and fasting LDL cholesterol levels. Thus, orlistat may represent another relatively unexplored pharmacological approach to cardiovascular risk pathologies (31).
EFFECT ON TRIGLYCERIDES
Our systematic review suggests that orlistat can help to reduce PHTGL in obese, dislipemic and type 2 diabetic patients. In our revision, we have identified nine out of a total of eleven studies where orlistat attenuates postprandial TGL response to an OFLT. Only two studies (25,26) did not find significant results. One possible reason could be that individuals studied were normolipemic, so the increase after OFLT was too small to detect statistical significant differences. Furthermore, in these two studies orlistat treatment was prescribed before the OFLT (during ten days) and this could have attenuated the effect.
PHTGL may contribute to the increased risk of cardiovascular disease since there is evidence that accumulation of the remnant particles is highly atherogenic (2,3). In addition, there is a weight-independent reduction in LDL-cholesterol in patients using orlistat, and it could be a good alternative for patients that do not tolerate statins (32). Hutton and Fergusson (33) reviewed 28 RCTs comparing orlistat to placebo during 6 months. The data showed a significant decrease in total cholesterol and LDL cholesterol with smaller decreases in serum TGL and HDL cholesterol.
Reitsma et al. (24) presented the first study about the effect of orlistat on OFLT, and speculated that the minor absorption of dietary fat with orlistat leads to a decreased formation of chylomicrons and chylomicron-remnants. The reason for the underlying mechanism could be that they are removed from circulation by the liver using the LDL receptor. Reduction in dietary fat absorption by orlistat may decrease the flux of cholesterol and TGL particles in the liver, resulting in the upregulation of hepatic LDL (34). Chylomicron-remnants are rich in cholesterol but poor in TGL, and that means a possible role in the process of atherogenesis.
Later, others authors (18-20) showed similar results in healthy volunteers. They studied other steps of the TGL metabolism. Suter et al. (18) evaluated the effect of orlistat on postprandial lipemia and lipoprotein particle subclass after the ingestion of moderate and high-fat meals in healthy volunteers. Mean change in large VLDL subclass concentration and mean VLDL size were significantly lower with orlistat. Large VLDL particles are preferentially metabolized to small, dense LDL particles which may have an increased atherosclerotic potential (37). But Suter et al. (18) were not able to discriminate between intestinal chylomicrons and hepatic VLDL. In two studies with Philippine healthy volunteers (19,20), orlistat abolished postprandial peak of TGL and VLDL after a single high-fat meal.
In a study with type 2 diabetic patients, Tan et al. (22) discovered a significant acute effect of a single dose of orlistat on remnant-like particles of cholesterol. PHTGL is an inherent feature of diabetic dyslipidemia, and is frequently found even in diabetic patients with normal fasting triglyceride due to the long residence time of chylomicron and VLDL remnants in circulation (38). Triglyceride-rich lipoprotein remnants are formed by lipoprotein lipase from intestinal chylomicrons in smaller and denser particles. These remnant particles are enriched in cholesteryl esters and poor in TGL. Some studies had shown that post-prandial changes in small remnant particles contribute to the severity of coronary heart disease in patients with type 2 diabetes mellitus (39,40).
EFFECT ON FREE FATTY ACIDS (FFA)
Tan et al. (22) also found a significant reduction in plasma FFA in patients with type 2 diabetes after treatment with orlistat which had not been previously reported. Di Somma et al. (26) observed the same results in obese patients with metabolic syndrome, but Sutter et al. (18), in healthy volunteers, and Damci et al. (23) did not find significant differences in type 2 diabetic patients.
Plasma FFA concentrations normally decrease in the early post-prandial period as the release of FFA from adipose tissue is suppressed by insulin and FFA derived from lipolysis of intestinal triglyceride-rich lipoproteins are taken up by the liver and re-esterified into triglyceride. Patients with type 2 diabetes have higher fasting and postprandial FFA (41). Orlistat could reduce FFA possibly by the decreased formation of chylomicrons, but not all authors have found the same results.
EFFECT ON GLUCAGON-LIKE PEPTIDE-1 (GLP-1)
In obese type 2 diabetic patients, orlistat treatment is associated with improved glycemic control in the long term (42) and also with a lower incidence of type 2 diabetes in individuals with prediabetes (16). The anti-hyperglycemic effect of orlistat has been attributed to weight loss associated with a decrease in insulin resistance. However, the improvement in postprandial glucose levels with orlistat could be related to the improvement of postprandial secretion of certain incretins, such as GLP-1, due to increased delivery of fats to the distal ileum. An increase of GLP-1 mediated by orlistat could lead to suppressed appetite and improvement in meal-stimulated insulin release.
However, in type 2 diabetic patients we have found three studies with conflicting results over the immediate effect of orlistat on the postprandial GLP-1 response. Damci et al. (23) found that a single dose of orlistat significantly enhanced the postprandial increase in GLP-1 and C-peptide levels and attenuated the postprandial rise in glucose and triglycerides in 29 type 2 diabetic patients.
Pilichiewicz et al. (43) studied, in 7 subjects with type 2 diabetes mellitus, the effect of adding orlistat to a liquid meal containing oil and glucose consumed in the left lateral decubitus position. They found that orlistat potentiates postprandial rises in plasma insulin but attenuates plasma GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) responses. Later, the same group presented another study with 8 type 2 diabetic patients (44), and they once more discovered that the inhibition of fat absorption by orlistat results in a more rapid gastric emptying and a diminished GLP-1 response.
The same group of healthy volunteers (45) showed that the increase in GLP-1 concentrations following a duodenal fat infusion is abolished by lipase inhibition with orlistat.
Sahin et al. (46) found in 16 obese non-diabetic patients that orlistat had no significant effect on postprandial GLP-1, glucose or insulin levels after a mixed meal, although GLP-1 and insulin peaks were delayed into the second postprandial hour after treatment with orlistat.
These contradictions regarding results could be explained by various factors like short life of plasma insulin or GLP-1, different type of hypoglycemic treatments or amount of fat in the OFLT. Furthermore, Beyson et al. (47) demonstrated differential stimulation of GLP-1 release by different types of oral fat (monounsaturated, olive oil; polyunsaturated, safflower oil; saturated, palm stearin). The failure to get this effect with statistical significance may be due to the fact that there was only a single postprandial sampling of blood in these studies (23,43-46).
In a repetitive administration of orlistat over a 10 day period, Di Somma et al. (26) found lower postprandial levels of plasmatic insulin in OFLT with orlistat, but they did not study GLP-1 levels. In our opinion, the results of Damci et al. (23) are more convincing because plasma half-life of C-peptide is 30 minutes, whereas insulin half-life is about 5 minutes.
EFFECT ON GASTRIC EMPTYING
On the other hand, orlistat has been reported to induce accelerated gastric emptying of fat and glucose, as O'Donovan studies (43-45) and other groups have demonstrated (48,49). Moreover, the lack of pancreatic lipase activity in cystic fibrosis with exocrine insufficiency results in an acceleration of gastric emptying due to a reduction in feedback inhibition from lipolytic products in the small intestine (50). Gastric emptying accounts for approximately 35% of the variance in initial postprandial blood glucose concentrations after a 75 g oral glucose load in both healthy subjects (51) and type 2 diabetes (52).
EFFECTION ON FASTING NORMOLIPEMIA AND PHTGL PATIENTS
There are patients with fasting normolipemia and PHTGL. Its prevalence has not been established but it is associated with metabolic syndrome components, like androgen obesity, dislipemia and insulin resistance. This kind of patients could represent the main target of therapy with orlistat. Turker et al. (27) found, in fasting normolipidemic obese women and PHTGL, that 12 weeks of treatment with orlistat plus a low calories diet was associated with a reduction in postprandial TGL, independent of reductions from baseline in weight, waist circumference and insulin resistance.
The prescription label of orlistat establishes that the pharmacologic effect of the drug is observed within 24-48 hours based on the amount of fecal fat excreted (35,36). We have identified in this systematic review 7 papers (18,24) that studied the acute effect of a single dose of orlistat on OFLT. It is important to note this fast effect because it is an expensive treatment and this test could select the responders' patients. OFLT is a test that examines responses to high-fat meal consumption (53) and it is indicated to diagnose PHTGL, in the same way that an oral glucose tolerance test is used for diabetes diagnosis. The association of orlistat to OFLT could offer double interest: diagnosis of PHTGL and orlistat potency in each patient.
Our present review presents several methodological limitations because of the heterogeneity of the studies. The absence of standardization of OFLT is the most important problem regarding its general use. Castro-Cabezas et al. (54) have proposed the autodetermination of diurnal capillary TGL after each meal. Moreover, non-fasting TGL has been used for screening and management of elevated levels of TGL in recent major guidelines (10,11). Secondly, the majority of the study presents a very small number of patients, so their results can only suggest tendencies. Thirdly, there is a very low number of studies for each group of patients, which are joined to the small number of patients in each study, making it impossible to perform a meta-analysis.
Conclusion
Despite these limitations, we can conclude that the positive and consistent findings of the included studies suggest that orlistat can help to reduce postprandial plasmatic triglyceride, especially in patients with postprandial hypertriglyceridemia related to obesity, dyslipidemia or type-2 diabetes.
References
1. Chan DC, Pang J, Romic G and G. Watts GF. Postprandial hypertriglyceridemia and cardiovascular disease: Current and future therapies. Curr Atheroscler Rep 2013;15:309. [ Links ]
2. Borén J, Matikainen N, Adiels M, Taskinen MR. Postprandial hypertriglyceridemia as a coronary risk factor. Clinica Chimica Acta 2014;431:131-42. [ Links ]
3. Pirillo A, Norata GD, Catapano AL. Postprandial lipemia as a cardiometabolic risk factor. Current Medical Research & Opinion 2014;30:1489-503. [ Links ]
4. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299-308. [ Links ]
5. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309-16. [ Links ]
6. Lindman AS, Veierod MB, Tverdal A, Pedersen JI, Selmer R. Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian counties study. Eur J Epidemiol 2010;25:789-98. [ Links ]
7. Langsted A, Freiberg JJ, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Nordestgaard BG. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: The Copenhagen City Heart study with 31 years of follow-up. J Intern Med 2011;270:65-75. [ Links ]
8. Varbo A, Nordestgaard BG, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Benn M. Nonfasting triglycerides, cholesterol, and ischemic stroke in the general population. Ann Neurol 2011;69:628-34. [ Links ]
9. Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, et al. Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration. Triglyceride-mediated pathways and coronary disease: Collaborative analysis of 101 studies. Lancet 2010;375:1634-9. [ Links ]
10. Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, et al. European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur Heart J 2011;32:1345-61. [ Links ]
11. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Circulation 2011;123:2292-333. [ Links ]
12. Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, et al. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr Vasc Pharmacol 2011;9:258-70. [ Links ]
13. Zhou YH, Ma XQ, Wu C, Lu J, Zhang SS, Guo J, et al. Effect of anti-obesity drug on cardiovascular risk factors: A systematic review and meta-analysis of randomized controlled trials. PLoS One 2012;7:e39062. [ Links ]
14. Lindgärde F, on behalf of the orlistat Swedish Multimorbidity Study Group. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: The Swedish Multimorbidity Study. J Intern Med 2000;248:245-54. [ Links ]
15. UK Multimorbidity Study Group. Randomized trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. Int J Clin Pract 2002;56:494-9. [ Links ]
16. Jarl ST, Boldrin MN, Hauptman J, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155-61. [ Links ]
17. Jadad AR1, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of randomized controlled trials: Is blinding necessary? Control Clin Trials 1996;17:1-12. [ Links ]
18. Suter PM, Marmier G, Veya-Linder C, Hänseler E, Lentz J, Vetter W, et al. Effect of orlistat on postprandial lipemia, NMR lipoprotein subclass profiles and particle size. Atherosclerosis 2005;180:127-35. [ Links ]
19. Gabriel FS, Samson CE, Abejuela ZR, Sicat-Gabriel PR, Sumpio JP, Zacarias MB, et al. Postprandial effect of orlistat on the peaking of lipid level after sequential high fat meals. Int J Endocrinol Metab 2012;10:458-63. [ Links ]
20. Abejuela ZR, Macaballug A, Sumpio J, Zacarias M, Mercado-Asis L. Orlistat abolishes postprandial lipid peaking. Int J Endocrinol Metab 2009;3:179-86. [ Links ]
21. Cintora H, González C, Machain M, Cintora F, Montero J. Efecto agudo de orlistat en la lipemia posprandial. Clin Invest Arterioscl 2003;15:99-105. [ Links ]
22. Tan KC, Tso AW, Tam SC, Pang RW, Lam KS. Acute effect of orlistat on post-prandial lipemia and free fatty acids in overweight patients with type 2 diabetes mellitus. Diabet Med 2002;19:944-8. [ Links ]
23. Damci T, Yalin S, Balci H, Osar Z, Korugan U, Ozyazar M, et al. Orlistat augments postprandial increases in glucagon-like peptide 1 in obese type 2 diabetic patients. Diabetes Care 2004;27:1077-80. [ Links ]
24. Reitsma JB, Castro Cabezas M, De Bruin TW, Erkelens DW. Relationship between improved postprandial lipemia and low-density lipoprotein metabolism during treatment with tetrahydrolipstatin, a pancreatic lipase inhibitor. Metabolism 1994;43:293-8. [ Links ]
25. Shepard TY, Jensen DR, Blotner S, Zhi J, Guerciolini R, Pace D, et al. Orlistat fails to alter postprandial plasma lipid excursions or plasma lipases in normal-weight male volunteers. Int J Obes Relat Metab Disord 2000;24:187-94. [ Links ]
26. Di Somma C, Rivellese A, Pizza G, Patti L, De Rosa A, Cipriano P, et al. Effects of short-term treatment with orlistat on growth hormone/insulin-like growth factor-I axis in obese post-menopausal women. J Endocrinol Invest 2011;34:90-6. [ Links ]
27. Turker I, Guvener Demirag N, Tanaci N, Uslu Tutar N, Kirbas I. Effects of orlistat plus diet on postprandial lipemia and brachial artery reactivity in normolipidemic, obese women with normal glucose tolerance: A prospective, randomized, controlled study. Curr Ther Res Clin Exp 2006;67:159-73. [ Links ]
28. Tzotzas T, Samara M, Constantinidis T, Tziomalos K, Krassas G. Short-term administration of orlistat reduced daytime triglyceridemia in obese women with the metabolic syndrome. Angiology 2007;58:26-33. [ Links ]
29. Agencia Española de Medicamentos y Productos Sanitarios AEMPS. Nota informativa de la reunión del Comité de Medicamentos de uso Humano (CMH), celebrada el 11 de marzo de 2014. [ Links ]
30. Halpern B, Halpern A. Safety assessment of FDA-approved (orlistat and lorcaserin) anti-obesity medications. Expert Opin Drug Saf 2015;14:305-15. [ Links ]
31. Swinburn BA1, Carey D, Hills AP, Hooper M, Marks S, Proietto J, et al. Effects of orlistat on cardiovascular disease risk in obese adults. Diabetes Obes Metab 2005;7:254-62. [ Links ]
32. Halpern A, Mancini MC, Suplicy H, Zanella MT, Repetto G, Gross J, et al. Latin-American trial of orlistat for weight loss and improvement in glycaemic profile in obese diabetic patients. Diabetes Obes Metab 2003;5:180-8. [ Links ]
33. Hutton B, Fergusson D. Changes in body weight and serum lipid profile in obese patients treated with orlistat in addition to a hypocaloric diet: A systematic review of randomized clinical trials. Am J Clin Nutr 2004:80:1461-8. [ Links ]
34. Eleftheriadou I, Grigoropoulou P, Katsilambros N, Tentolouris N. The effects of medications used for the management of diabetes and obesity on postprandial lipid metabolism. Current Diabetes Reviews 2008;4:340-56. [ Links ]
35. Zhi J, Melia AT, Guerciolini R, Chung J, Kinberg J, Hauptman JB, et al. Retrospective population-based analysis of the dose-response (fecal fat excretion) relationship of orlistat in normal and obese volunteers. Clin Pharmacol Ther 1994;56:82-5. [ Links ]
36. Ahnen DJ, Guerciolini R, Hauptman J, Blotner S, Woods CJ, Wargovich ML. Effect of orlistat on fecal fat, fecal biliary acids, and colonic cell proliferation in obese subjects. Clinical Gastroentorology and Hepatology 2007;5:1291-9. [ Links ]
37. Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol 2002;22:1175-80. [ Links ]
38. Nero N, Syvanne M, Taskinen MR. Postprandial lipid metabolism in diabetes. Atherosclerosis 1998;141:S53-55. [ Links ]
39. Mero N, Malmstrom R, Steiner G, Taskinen MR, Syvanne M. Postprandial metabolism of apolipoprotein B-48- and B-100-containing particles in type 2 diabetes mellitus: Relations to angiographically verified severity of coronary artery disease. Atherosclerosis 2000;150:167-77. [ Links ]
40. Masuoka H, Kamei S, Wagayama H, Ozaki M, Kawasaki A, Tanaka T, et al. Association of remnant-like particle cholesterol with coronary artery disease in patients with normal total cholesterol levels. Am Heart J 2000;139:305-10. [ Links ]
41. Cooper MB, Tan KCB, Hales CN, Betteridge DJ. Postprandial lipid metabolism and b-cell function in non-insulin-dependent (type 2) diabetes mellitus after a mixed meal with a high fat content. Diabet Med 1996;13:816-27. [ Links ]
42. Hollander PA, Elbein SC, Hirsch IB, Kelley D, McGill J, Taylor T. Role of orlistat in the treatment of obese patients with type 2 diabetes: A 1-year randomized double-blind study. Diabetes Care 1998;21:1288-94. [ Links ]
43. Pilichiewicz A, O'Donovan D, Feinle C, Lei Y, Wishart JM, Bryant L, et al. Effect of lipase inhibition on gastric emptying of, and the glycemic and incretin responses to, an oil/aqueous drink in type 2 diabetes mellitus. J Clin Endocrinol Metab 2003;88:3829-34. [ Links ]
44. O'Donovan D, Horowitz M, Russo A, Feinle-Bisset C, Murolo N, Gentilcore D, et al. Effects of lipase inhibition on gastric emptying of, and on the glycaemic, insulin and cardiovascular responses to, a high-fat/carbohydrate meal in type 2 diabetes. Diabetologia 2004;47:2208-14. [ Links ]
45. O'Donovan D, Feinle-Bisset C, Wishart J, Horowitz M. Lipase inhibition attenuates the acute inhibitory effects of oral fat on food intake in healthy subjects. British Journal of Nutrition 2003;90:849-52. [ Links ]
46. Sahin M, Tanaci N, Yucel M, Tutuncu NB, Guvener N. The effect of single-dose orlistat on postprandial serum glucose, insulin and glucagon-like peptide-1 levels in nondiabetic obese patients. Clin Endocrinol (Oxf) 2007;67:346-50. [ Links ]
47. Beysen C, Karpe F, Fielding BA, Clark A, Levy JC, Frayn KN. Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia 2002;45:1533-41. [ Links ]
48. Borovicka J, Schwizer W, Guttmann G, Hartmann D, Kosinski M, Wastiel C, et al. Role of lipase in the regulation of postprandial gastric acid secretion and emptying of fat in humans: A study with orlistat, a highly specific lipase inhibitor. Gut 2000;46:774-81. [ Links ]
49. Demarchi B, Vos R, Deprez P, Janssens J, Tack J. Influence of a lipase inhibitor on gastric sensitivity and accommodation to an orally ingested meal. Aliment Pharmacol Ther 2004;19:1261-8. [ Links ]
50. Long WB, Weiss JB. Rapid gastric emptying of fatty meals in pancreatic insufficiency. Gastroenterology 1974;67:920-5. [ Links ]
51. Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1996;36:857-62. [ Links ]
52. Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med 1996;37:1643-8. [ Links ]
53. Mihas C, Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, et al. Diagnostic value of postprandial triglyceride testing in healthy subjects: A meta-analysis. Current Vascular Pharmacology 2011;9:271-80. [ Links ]
54. Castro Cabezas M, Halkes CJM, Meijsson S, Van Oostrom AJHHM, Erkelens DW. Diurnal triglyceride profiles: A novel approach to study triglyceride changes. Atherosclerosis 2001;155:219-28. [ Links ]
55. Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. NN8022-1807 Study Group. Effects of liraglutide in the treatment of obesity: A randomized, double-blind, placebo-controlled study. Lancet 2009;374:1606-16. [ Links ]
![]() Correspondence:
Correspondence:
Alejandro Sanz-Paris.
Unit of Dietetics and Nutrition.
Department of Endocrinology and Nutrition.
Hospital Universitario Miguel Servet.
Paseo Isabel la Católica, 1-3.
50009 Zaragoza, Spain
e-mail: sanzparisalejandro@gmail.com
Received: 25/09/2015
Accepted: 04/02/2016