My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.34 n.1 Madrid Jan./Feb. 2017
https://dx.doi.org/10.20960/nh.996
Euterpe edulis effects on cardiac and renal tissues of Wistar rats fed with cafeteria diet
Efectos de Euterpe edulis en los tejidos cardiacos y renales de ratas Wistar alimentadas con dieta de cafetería
Rodrigo de Barros Freitas1, Fernanda Araujo Melato1, Jerusa Maria de Oliveira2, Daniel Silva Sena Bastos2, Raisa Mirella Cardoso1, João Paulo Viana Leite3 and Luciana Moreira Lima1
1Departamento de Medicina e Enfermagem,
2Departamento de Biologia Geral, and
3Departamento de Bioquímica e Biologia Molecular.
Universidade Federal de Viçosa. Minas Gerais, Brasil
Agradecimentos: Fundação de Amparo à Pesquisa de Minas Gerais - FAPEMIG APQ-01877-13.
ABSTRACT
Introduction: This study's objective was to evaluate the antioxidant and toxic effects of E. edulis on cardiac and renal tissues of Wistar rats fed with cafeteria diet.
Methods: Catalase (CAT), glutathione-S-transferase (GST), superoxide dismutase (SOD) and malondialdehyde (MDA) were measured in cardiac muscle and renal tissue of 60 animals, which were randomly assigned for 10 equal groups. Half of the rats were fed with cafeteria diet and the other half with commercial chow, combined or not to E. edulis lyophilized extract, E. edulis deffated lyophilized extract or E. edulis oil. Data were evaluated using ANOVA, followed by the Student-Newman-Keuls test.
Results: Data showed a significant increase of CAT activity in cardiac tissue of animals from the groups fed with cafeteria diet associated to E. edulis lyophilized extract at 5%, E. edulis lyophilized extract at 10% and E. edulis deffated lyophilized extract at 10%. In addition, the same result was found in animals from the groups fed with commercial chow and commercial chow combined with E. edulis lyophilized extract at 10% in comparison to the group fed exclusively with cafeteria diet. GST and SOD enzyme activity showed significant increase in the heart tissue of animals nourished with commercial chow when compared to the groups fed with cafeteria diet. On the other hand, there were no significant differences enzymatic levels in renal tissues.
Conclusion: The oil and the extract of E. edulis had an important role promoting an increase of antioxidant enzymes levels in cardiac muscle, which prevent the oxidative damage resulting from the cafeteria diet in Wistar rats. There were no evidenced signs of lipid peroxidation in renal or in cardiac tissue of the animals studied, indicating that the E. edulis use did not promote any increase in malondialdehyde cytotoxic products formation. This show that both E. edulis oil and extracts evaluated in this study were well tolerated in the studied doses.
Key words: Euterpe edulis. Cafeteria diet. Antioxidant enzymes. Anthocyanins.
RESUMEN
Introducción: el objetivo de este estudio fue evaluar los efectos antioxidantes y tóxicos de E. edulis en los tejidos cardiacos y renales de ratas Wistar alimentadas con dieta de cafetería.
Métodos: catalasa (CAT), glutatión-S-transferasa (GST), superóxido dismutasa (SOD) y malondialdehído (MDA) se midieron en el músculo cardiaco y el tejido renal de 60 animales, que fueron asignados aleatoriamente para 10 grupos iguales. La mitad de las ratas fueron alimentadas con dieta de cafetería y la otra mitad con ración comercial, combinados o no con E. edulis extracto liofilizado, E. edulis GMD obtenidas de extracto liofilizado o aceite de E. edulis. Los datos se evaluaron mediante ANOVA, seguido por el test de Student-Newman-Keuls.
Resultados: los datos mostraron un aumento significativo de la actividad de CAT en el tejido cardiaco de los animales de los grupos alimentados con dieta de cafetería asociada a E. edulis extracto liofilizado en un 5%, E. edulis extracto liofilizado en un 10% y E. edulis GMD obtenidas de extracto liofilizado de 10%. Además, el mismo resultado se encuentra en los animales de los grupos alimentados con chow chow comercial y comercial combinado con extracto liofilizado E. edulis en 10% en comparación con el grupo alimentado exclusivamente con dieta de cafetería. La actividad de GST y la enzima SOD mostró un aumento significativo en el tejido del corazón de los animales alimentados con pienso comercial en comparación con los grupos alimentados con dieta de cafetería. Por otro lado, se observaron diferencias significativas en los niveles enzimáticos en los tejidos renales.
Conclusión: el aceite y el extracto de E. edulis tuvieron un papel importante al promover un aumento de los niveles de enzimas antioxidantes en el músculo cardiaco, que previenen el daño oxidativo resultante de la dieta de cafetería en ratas Wistar. Los signos de la peroxidación lipídica evidenciados en los riñones o en el tejido cardiaco de los animales estudiados indican que el uso de E. edulis no promovió ningún aumento en la formación de productos citotóxicos malondialdehído, un marcador reconocido de la acción de los radicales libres.
Palabras clave: Euterpe edulis. Dieta de cafetería. Enzimas antioxidantes. Antocianinas.
Introduction
Under physiological conditions, the toxic effects of reactive oxygen species (ROS) may be blocked by scavenging enzymes, such as superoxide dismutase (SOD) and catalase (CAT) (1). However, when ROS production exceeds the cellular oxidative stress handling system, functional and constitutional integrity of tissues can be affected, which can be detected in a vast range of metabolic and inflammatory disease states, including obesity and dyslipidemia (2,3). Oxidative stress mediates apoptosis in the cardiac tissue, activate signaling kinases and transcription factors evolved on cardiac hypertrophy. It is also part of a pathophysiological mechanism of cardiac tissue remodeling, which is responsible for the installation and evolution of heart failure. On the other hand, it can promote renal cells apoptosis and ageing, declined regenerative capacity of cells and fibrosis, which are part of the pathogenesis of chronic kidney failure.
Fruits and vegetables have a wide spectrum of possibly cardio and renal protective nutrients, such as vitamins and a range of non-nutrient phytochemicals, for example carotenoids and polyphenols. However, the effects of these components in the cardiovascular and oxidative stress system are far from clear (4). Many epidemiological studies showed an inverse association between dietary intake of vitamin E and cardiovascular disease (5), and there are evidences fruits containing large concentrations of procyanindins, anthocyanin and flavonols are more efficient at reducing cardiovascular risk, they inhibit platelets aggregation, have anti-hypertensive effects and increase endothelial-dependent vasodilatation. In addition, red grape juice, which main poliphenolic components are anthocyanins, has some role in platelet aggregation inhibition. Furthermore, polyphenols protection in the kidney has been reported both in humans and rodents in red wine administration, associated with the increased antioxidant capacity of plasma. In this case, the major two groups of phenolic compounds are anthocyanins and flavonoids (6).
Euterpe oleracea is the best known and most appreciated Brazilian palm heart. This palm heart has a high antioxidant capacity and it is largely used as food, demonstrating anti-inflammatory activity and hypocholesterolemic effects (7). At the same concentration, Euterpe edulis pulp has around half of the polyphenols found in Euterpe oleracea pulp. For this reason, in this study we tested the Euterpe edulis possible antioxidant and toxic effects in cardiac and renal tissues in rats.
Material and methods
EXTRACTS OBTAINING
Ninety kilograms of fruits were immersed in warm water (40-60 oC) for 15 minutes to facilitate the softening of the epicarp and mesocarp, followed by removal of this water. The pulps were separated in industrial depulper with addition of deionized water in a 2:1 ratio with the result of E. edulis, respectively. The pulp was passed through a fine mesh screen and lyophilized (Lyophilizer Liotop, Liobras®), which was named LEE. Part of this extract was stored in a cold storage, around -20 oC for posterior analysis, while the other part was submitted to a process of oil removal, using Soxhlet (Quimis®) extraction in ethyl ether anhydrous. After twelve hours of extraction, the lipophilic solution was taken to the rotary evaporator in order to remove the ether completely, which resulted in oil from the fruits of E. edulis. After complete removal of the residual ether, which was performed in air circulation environment, protected from light and at room temperature, the extract in the interior of the Soxhlet cartridge was named defatted extract. All the extract were packed in nitrogen environment and stored at -20 oC in amber bottles until they were used.
IN VIVO EXPERIMENTS
The study was approved by the Ethics Committee for Animal Use (ECAU)/UFV, through the process number 60/2012. Sixty mice (Rattus norvergicus albinus) of Wistar lineage, ageing four weeks, were acclimated for seven days after received to the vivarium, then they were fed with cafeteria diet for 30 days. In addition to the cafeteria diet, they received extracts of E. edulis during twenty days, with a total of fifty days of experiment. All procedures were performed in light controlled conditions (twelve hours cycles of light/dark), temperature controlled conditions (21 ± 2 oC) and relative humidity controlled conditions (60%).
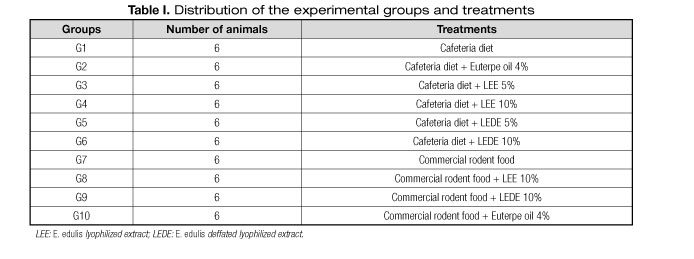
The animals were assigned to ten experimental groups containing six animals each and randomly distributed according to table I.
The composition of the cafeteria diet is described in table II, provided ad libitum throughout the experiment. The diet was prepared in aseptic environment with industrial mixer assistance in order to homogenize the mixture.
When the experimental period ended, the animals were euthanized with hypovolemic shock, which was realized by puncturing the abdominal aorta, under anesthesia in alothane chamber. After dissection cardiac muscle and kidney was excised immediately, the adhering tissue was cleared off, and they were weighed and frozen at -80 oC. Small portions of the kidney (50 mg) and cardiac muscle (50 mg) were homogeneized on K-phosphate buffer 50 mM pH 7.4 (500 µL) and centrifuged (13,8 g at 4 oC for por 10 minutes) for SOD, CAT and GST determinations, along with lipid peroxidation products.
ANALISYS OF ANTIOXIDANT ENZYMES
CAT activity of this enzyme was measured in the supernatant by the decreased rate of hydrogen peroxide (H2O2) (10 mmol/L) in a spectrophotometer at 240 nm, registered at interval of 60s (8), in a cuvette containing aliquots of supernatant, 50 mM K-phosphate buffer pH 7.0, and freshly prepared 10 mM hydrogen peroxide. The molar extinction coefficient of hydrogen peroxide at 240 nm is 36 mol/L/cm, which was used for the calculations. Catalase activity is expressed as U CAT/ mg protein.
SOD activity was measured from the supernatant using an ELISA reader at 570 nm based on the ability of this enzyme to catalyze the reaction of the superoxide and hydrogen peroxide, and thereby decreasing the auto-oxidation rate of pyrogallol (9). Aliquots of supernatant were added to a well containing a Na-phosphate buffer 50 mM pH 7.0, pyrogallic acid (100 µM), MTT 3-(4,5-dimethylthiazol-2-H)-2,5-diphenyltetrazolium bromide (1,25 mM), and Dimethyl sulfoxide (DMSO). SOD activity is expressed as U SOD/mg protein.
GST was measured through the formation of GSH conjugate, 2.4- dinitrobenzene, and estimated by the change in absorbance at 340 nm for 60 s in a cuvette containing 50 mM K-phosphate buffer pH 7.0, 1-cloro-2,4-dinitrobenzeno (0.1 M), reduced glutathione (GSH) (0.1 M) and aliquots of supernatant. Conjugate formation occurs spontaneously on the substrate, 1-chloro-2.4-dinitrobenzene (CDNB), in a non-enzymatic reaction, and is accelerated by the activity of GST enzymes. One unit (U) of the enzyme activity is the amount of GST enzyme that forms 1 mol of GSH conjugate, 2.4-dinitrobenzene, per minute. The molar extinction coefficient of CDNB at 340 nm is 9.6/mM/cm, which was used for the calculations (10). The GST activity is expressed as µmol/min/g.
LIPID PEROXIDATION ESTIMATION ASSAY
Substances that react to thiobarbituric acid are mostly products of lipid peroxidation, and malondialdehyde (MDA) is an important marker to monitor lipid peroxidation rate. The thiobarbituric acid reactive substance (TBARS) solution [15% trichloroacetic acid (TCA), 0.375% thiobarbituric acid, and HCL 0.25 N] were added to aliquots of supernatant and were in a water bath for 15 min, cooled, and centrifuged at 0.9 g. The supernatant was used for estimation at 535 nm in a spectrophotometer (11). The molar extinction coefficient of TBA-MDA complex at 535 nm is 1.56/M/cm, was used for calculations. Absorbances is expressed as equivalent to MDA nmol/mg protein.
TOTAL PROTEIN
The total protein content in SOD, CAT, and TBARS homogenates was measured in 100 μL from each homogenate that had 1 mL of ABC solution (A: 30 g of Na2CO3 + 4 g of de NaOH; B: CuSO4 2%; C: Sodium tartrate 4%) added and completed with H2O until 1,000 mL. The final solution was vortexed and remained sitting for 10 min, when 100 μl from Folin-Ciocaulteu solution (1:2) was added. After allowing 10 more min of rest, samples were red using an ELISA reader at 700 nm (12). The concentrations of total protein were determined using bovine serum albumin as a standard curve.
STATISTICAL ANALYSIS
The minimum size of the sample was defined using an estimated variance (s2) of a sample pilot and another of the literature. The formula proposed by Callegari-Jacques (2003) (13) and Cochran (1977) (14) was used for the calculation. It was possible to verify statistical differences with a 5% level of significance, with a minimum number of five animals each group.
The data were expressed as means ± standard error of mean (mean ± SEM). The normality of data distribution was verified using the D'agostino-pearson test. Data were submitted to the ANOVA test followed by the Student-Newman-Keuls (SNK) post hoc test for multiple comparisons. All the tests were performed using the GraphPad Prism 6.0 statistical software program (GraphPad Software, Inc, CA, USA) and statistical significance was established at p < 0.05.
Results
Tables III and IV present the values obtained for CAT, GST, MDA and SOD in cardiac muscle and renal tissue of animals studied in each group, respectively. Significant differences were observed only in cardiac tissue for the parameters, CAT, SOD and GST. In renal tissue no significant differences were observed between the groups for the studied parameters.
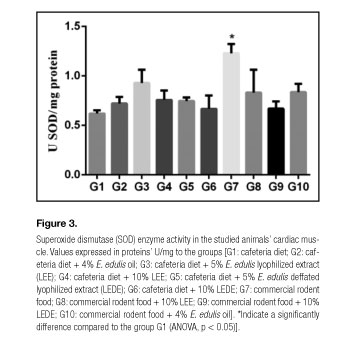
Figures 1, 2 and 3 present the values of CAT, GST and SOD in the studied animals' cardiac muscles. Compared to the group G1, the groups G3, G4, G5, G6, G7 and G8 presented significantly elevated CAT values; the groups G3, G4, G6, G7 and G8 presented considerably higher GST values; and the group G7 exhibited remarkably greater SOD values.
Discussion
The main finding of this study is that the cafeteria diet may have inhibited the expression of antioxidant substances in the studied animals' cardiac muscles compared with animals fed with commercial chow. On the other hand, the E. edulis lyophilized extract at 5% and at 10% did not inhibit the CAT expression in the studied rats' cardiac tissues; the same way the E. edulis oil at 4% did not inhibit the GST expression in these tissues.
The E. edulis contains high concentrations of antioxidant components, such as polyphenols and monomeric anthocyanins (15,16),- which have a large antioxidant potential and contribute to prevent cardiovascular and renal damage. They act inhibiting LDL cholesterol and platelet aggregation, among other mechanisms not fully elucidated.
Mammals are equipped with a variety of antioxidant mechanisms, which components are able to decrease or inhibit oxidation, even at concentrations lower than their biochemical substrate. Among these mechanisms, this study evaluated some endogenous enzymes, such as SOD, which function is to convert superoxide anions in H2O2, and CAT, which have important roles converting H2O2 in H2O and O2, and GST; these enzymes levels decrease is one of the factors that aggravate the oxidative damage caused by ROS. In addition, the study evaluated MDA, a final product of the lipid peroxidation and considered important to demonstrate oxidative stress (17,18).
Data showed a significantly increase in CAT enzyme activity in animals from groups that received commercial chow associated to E. edulis lyophilized extract 10%, in comparison with animals fed only with cafeteria diet (Table III, Fig. 1). On the other hand, CAT activity has shown a statistically significant decrease in the animals nourished exclusively with cafeteria diet compared with animals fed with commercial chow. This result indirectly suggests an increase in oxidative stress, related to the high fat diet. Noeman et al. (2), proposed mechanisms to explain the antioxidant enzymes decrease during the development of obesity in rats: the induced hypertriglyceridemia may increase the free fatty acids bioavailability, leading to a higher lipid peroxidation. Consequently, the endogenous enzymes may be inactivated due to cross-linking with MDA. In addition, the attempts to fight the free radicals can cause an accelerated consume and depletion of the enzymatic supplies.
Furthermore, the significantly higher CAT values found in the groups fed with cafeteria diet combined to E. edulis lyophilized extract at 5% (G3), E. edulis lyophilized extract at 10% (G4) and E. edulis defatted lyophilized extract at 10% (G6) compared with the group fed only with cafeteria diet (Table I), suggests a decreased level of oxidative stress in heart tissues, probably because of E. edulis extract association. Besides, the average CAT values found in group fed with commercial chow associated to E. edulis lyophilized extract at 10% (G8) were greater than the enzymatic average values found in the group provided only with commercial chow (G7), even though there was no statistical significance. These findings may indicate an antioxidant activity increase in cardiac tissues of animals nourished with E. edulis lyophilized extract at 10% regardless using commercial chow or cafeteria diet. Nonetheless, further studies are needed to elucidate with reliability the correlation between the E. edulis lyophilized extract at 10% and the CAT expression increase in heart tissues of Wistar rats provided with different diets.
This study demonstrated a GST enzymatic activity significant increase in the cardiac muscles of animals from all the groups fed with commercial chow (G7, G8, G9, G10) in comparison with the group provided with cafeteria diet (G1), regardless the association of E. edulis (Table III, Fig. 2). These findings are supported due to the SOD expression significant increase found in animals from the group nourished only with commercial chow (G7) compared with the group fed only with cafeteria diet (G1) (Table III, Fig. 3). Our results are in agreement with the results of Mehra et. al. (19), who showed a SOD levels significant decrease in high fat diet fed rats.
In the present study, only the E. edulis oil at 4% led to a GST activity significant increase in cardiac muscles of animals fed with cafeteria diet (G2). GST belongs to a group of enzymes which function is catalyze the glutathione conjugation with a range of molecules, having a key role in intracellular detoxification mechanisms of endo and xenobiotic compounds (10) and protecting cells against chemical toxicity and stress. Thus, data obtained for GST may corroborate the results found for CAT regarding the -E. edulis benefits in decreasing oxidative stress in heart tissue, in this case considering the oil at 4%.
As reported in table IV, there were no significant differences in the studied enzymes activity in renal tissues of animals from any assessed group. There are mechanisms proposed to explain the role of oxidative stress in renal damage of obese rats: the high-fat diet may lead to changes in renal lipid metabolism due to a local imbalance between lipolysis and lipogenesis. The same way, systemic metabolic changes may lead to a perirenal accumulation of adipose tissue, which penetrates the renal medullary sinuses therefore increasing the intrarenal pressure. The elevated pressure damages the renal cells, releasing cytokines, particularly tumor necrosis factor alpha (TNF-α), which produce ROS from tissues, consequently increasing the lipid peroxidation in the renal tissue. Nonetheless, the cafeteria diet effects in the obesity induction process in rats are subtle and cumulative, requiring a minimum 10 weeks period to induce obesity. Although the hypertriglyceridemia caused by the cafeteria diet is alone a contributing factor to increase the bioavailability of free fatty acids, the present study was not conducted in a length of time considered sufficient to induce obesity in the rats. Therefore, we are unable to rule out the hypothesis that it has somehow influenced the results found in renal tissues. On the other hand, the heart tissue uses a large amount of fatty acids and glucose as energetic substrate and the final oxidation of these fuels occurs in the mitochondria by aerobic mechanisms, which justify the cardiac tissue susceptibility to oxidative stress.
Data from the present study showed no MDA levels significant differences either in renal or in cardiac tissues (p > 0.05), as shown in tables III and IV. This may indicate that there were no signs of lipid peroxidation or toxicity induced by free radicals formation, even with the extract containing the higher concentration of anthocyanin. Therefore, both E. edulis oil and extracts evaluated in this study were well tolerated in the studied doses.
It is important to emphasize that there is a limited number of studies analyzing the possible effects of E. edulis in oxidative stress in vivo, which makes difficult to compare our results with others. The present study suggests a decrease of oxidative stress levels in cardiac tissues of animals fed with E. edulis, in disagreement with the results of Castro et al. (20), who found no significant difference in the antioxidant enzymes levels. Castro et al. (20) propose that the amount of acai associated to the animals' diets were insufficient, probably due to the concentration used or the extraction protocol. Therefore, this study presents unpublished results about the E. edulis, which antioxidant effects in cardiac muscle had not yet been demonstrated.
Conclusion
Both E. edulis oil and extract had important roles increasing the expression of antioxidant enzymes in cardiac muscle of the studied animals, preventing the possible oxidative damage from the cafeteria diet in Wistar rats. There were no signs of lipid peroxidation neither in renal tissue nor in cardiac muscle of the studied animals, indicating that the E. edulis use did not increase the production of the cytotoxic product malondialdehyde. This show that both E. edulis oil and extracts evaluated in this study were well tolerated in the studied doses.
References
1. Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 2011;301(6):H2181-H2190. [ Links ]
2. Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetology & Metabolic Syndrome 2011;3(1):17. DOI:10.1186/1758-5996-3-17. [ Links ]
3. Small DM, Coombes JS, Bennett N, et al. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 2012;17(4):311-21. [ Links ]
4. Chong MFF, Macdonald R, Lovegrove A. Fruit polyphenols and CVD risk: a review of human intervention studies. Brit J Nutr 2010;104(3):S28-S39. [ Links ]
5. Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radical Bio Med 2014;72:76-90. [ Links ]
6. Rodrigo R, Miranda A, Vergara L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin Chim Acta 2011;412(5-6):410-24. [ Links ]
7. Felzenszwalb I, Marques MRC, Mazzei JL, Aiub CAF. Toxicological evaluation of Euterpe edulis: a potential superfruit to be considered. Food Chem Toxicol 2013;58:536-44. [ Links ]
8. Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121-6. [ Links ]
9. Dieterich S, Bieligk U, Beulich K, et al. Gene expression of antioxidative enzymes in the human heart: Increased expression of catalase in the end-stage failing heart. Circulation 2000;101:33-9. [ Links ]
10. Keen JK, Habig WH, JaKobi WB. Mechanism for the several activities of the glutathione-S-transferases. J Biol Chem 1976;251(20):6183-88. [ Links ]
11. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in Enzymol 1978;52:302-10. [ Links ]
12. Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75. [ Links ]
13. Callegari-Jacques SM. Bioestatística: princípios e aplicações. Porto Alegre: Artmed; 2007. [ Links ]
14. Cochran WG. Sampling techniques. New York: Wiley; 1977. [ Links ]
15. Cardoso LM, Novaes RD, Castro CA, et al. Chemical composition, characterization of anthocyanins and antioxidant potential of Euterpe edulis fruits: applicability on genetic dyslipidemia and hepatic steatosis in mice. Nutr Hosp 2015;32(2):702-9. [ Links ]
16. Novello AA, Conceição LL, Dias MMS, et al. Chemical characterization, antioxidant and antiatherogenic activity of anthocyanin-rich extract from Euterpe edulis Mart. in mice. J Food Nutr Res 2015;54:101-12. [ Links ]
17. Hopps E. Noto D, Caimi G, Averna M. A novel component of the metabolic syndrome: the oxidative stress. Nutr Metab Cardiovas 2010;20(1):72-7. [ Links ]
18. Afonso V, Champy R, Mitrovic D, et al. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine 2007;74(4):324-9. [ Links ]
19. Mehra P, Koul A, Bansal D. Studies on antioxidante role of (+)-catechin hydrate in high sucrose hi fat diet induced oxidative stress. Am J Biomed Sci 2013;5(2):161-70. [ Links ]
20. Castro CA, Natali AJ, Cardoso LM, et al. Aerobic exercise and not a diet supplemented with jussara açaí (Euterpe edulis Martius) alters hepatic oxidative and inflammatory biomarkers in ApoE-deficient mice. Br J Nutr 2014;112(3):285-94. [ Links ]
![]() Correspondence:
Correspondence:
Rodrigo de Barrios Freitas.
Departamento de Medicina e Enfermagem.
Universidade Federal de Viçosa.
Avenida Peter Henry Rolfs, s/n.
Campus Universitário.
Viçosa, Minas Gerais.
36570-900, Brasil
e-mail: rodrigodebarrosfreitas@yahoo.com.br
Received: 14/11/2015
Accepted: 16/11/2015