INTRODUCTION
Discussions on the benefits, harms, and limitations of the administration of medication via enteral feeding tubes (EFTs) have been held to ensure its efficacy, safety, and, above all, effectiveness and convenience for patients. Although parenteral administration ensures a high degree of absorption, it poses potentially high risks of complications and discomfort, is costly, and is not commonly used in long-term treatments 1-5. Transdermal, sublingual, rectal, and topical routes are limited to certain drugs; the oral route is a good alternative but it is not always available 6.
In particular, drug administration via EFTs is a challenge in clinical practice due to the scarce information about its use and the lack of commercially-available adequate formulations 4,7. Among other aspects, drug administration via EFTs carries a considerable risk of prescription incompatibility 3,6; in many situations, the administration of a drug via EFTs requires transformation of the drug's original physical properties, which can have implications for its effectiveness and safety 8. Additionally, such formulations must be prepared immediately before administration 1.
The dissolution or suspension of a solid dosage form in a compatible vehicle may require crushing; therefore, the chemical and physicochemical properties of the drug and its starting formulation, which determine its stability and pharmacokinetic profile, must be well known to avoid compromising the treatment's effectiveness and safety 3,5,9,10.
A study by Mota et al. 6 showed that it is difficult for the nursing team to administer drugs via EFTs. Most of the team members reported using metal, wood, or plastic mortars to crush solid dosage forms; the use of mortars may result in the loss of drug fragments in the mortars, potential reactions between the prescribed dosage form and the mortar material, and potential drug-drug interactions if the mortars are not washed thoroughly between each use.
The administration of medication via EFTs is a common practice but often done without adequate technical criteria. Enhancing the knowledge of practitioners involved in the care of patients might prevent problems with the efficacy and safety of pharmacological treatments and avoid inconveniencing the patients or causing problems with their diets. Nurses, nutritionists, and physicians should be encouraged to work with pharmacists to determine the best pharmacological management of patients receiving enteral nutrition 3,6,9-15.
When the oral route is unavailable and it is infeasible and/or inconvenient to use the parenteral route, the medication should be administered via EFTs 10. In fact, although the use, safety, and effectiveness of many drugs administered via EFTs are not well established, drug administration via EFTs has been used routinely in clinical practice due to a lack of options 16.
This study aimed to present a method of drug delivery via EFTs as a safe and convenient proposal that is suitable for use by inpatients.
MATERIAL AND METHODS
SETTING
The study took place in a public secondary care hospital accredited with level II accreditation by the National Accreditation Organization (Organização Nacional de Acreditação ONA). The hospital is located in the city of Fortaleza, Ceará, north-eastern Brazil.
EVALUATION OF STANDARDIZED ORAL SOLID MEDICATIONS
We analysed all the standardized oral solid medications (tablets, capsules, and dragees) used in the hospital where the study took place. There were 108 solid dosage forms that were not commercially available in liquid forms in Brazil but could be prescribed for administration via EFTs.
DISPERSION OF SOLID DOSAGE FORMS
All 108 oral solid medications were selected for evaluation of the transformation of the solid dosage form into a liquid form, which is the only form that can be administered through a feeding tube in the proposed method. Hard capsules were opened to assess the dispersion of their internal contents (powders) in water.
For the evaluation of the dispersion of the medications selected, the following steps were taken:
The medication was drawn into the oral syringe;
10 mL of mineral water were drawn into the syringe;
After mixing the medication with the water, the dispersion time of the medication was measured to assess its adequacy for EFT administration;
The time (minutes) that the medication took to fully disperse was recorded on a spreadsheet;
We also recorded visual assessments after 1, 5, 10, and 20 minutes as the feasibility cut-off value for dispersion in the dispenser; medications that dispersed in 20 minutes or less were considered feasible.
Standardized medications intended for administration via EFTs were selected according to their dispersion times and analysed for any contraindications against EFT administration and the need for crushing prior to dispersion. Medications whose dispersion could be visually perceived and whose content could be expelled without occluding the oral syringe were considered "satisfactory".
STUDY ON THE SAFETY OF THE USE OF ORAL SYRINGES
The safety of the extemporaneous preparations obtained from the dispersion of the solid dosage forms in the oral syringes was also studied. Given the high risk of administering these formulations through intravenous devices, oral syringes and parenteral syringes and their connections to EFT and intravenous devices were compared.
Regarding the safety analysis, the following parameters were evaluated: connection to infusion set, stopcock, and extension set; connection to venous access (central and peripheral); connection to the feeding tube; graduation; and risk of occlusion, which was estimated by assessing EFT diameter.
Statistical analysis
The results obtained from the evaluation of the dispersion time of tablets were analysed using descriptive statistics and a Microsoft Excel 2010 database.
ETHICAL APPROVAL
The study was approved by the Research Ethics Committee of the Federal University of Ceará upon submission of the research project to the Plataforma Brasil system. The study was approved under Opinion No. 507.830 and CAAE No. 21180413.1.0000.5054. The researchers had no conflicts of interest in performing this research and it did not pose any risks to the patients.
RESULTS
DISPERSION OF SOLID DOSAGE FORMS
Of the 108 solid medications dispersed in oral syringes with 10 mL of mineral water, only one needed a volume of water greater than 10 mL to disperse: the tablet provided by the Brazilian government to treat tuberculosis, which contained rifampicin, isoniazid, pyrazinamide, and ethambutol.
Dispersion occurred immediately or took up to one minute in 25% (n = 27) of the items tested; two to five minutes in 21.3% (n = 23); six to ten minutes in 15.7% (n = 17); and eleven to twenty minutes in 13.9% (n = 15). Thus, 75.9% (82 items) of the analysed medications were classified as capable of being dispersed in water in the oral syringe for further administration via EFTs without the need for crushing.
Tablets without satisfactory dispersion, in terms of quality and dispersion time, were indicated for crushing by a mortar and pestle. After crushing, these medications were placed into the oral syringe with mineral water to evaluate the dispersion. A total of 9.2% (n = 10) of the items tested did not disperse, even after crushing; these medications were classified as "inappropriate" for administration via EFTs (Table I), given that these medications could cause tubal occlusion or blockage and/or generate drug ineffectiveness.
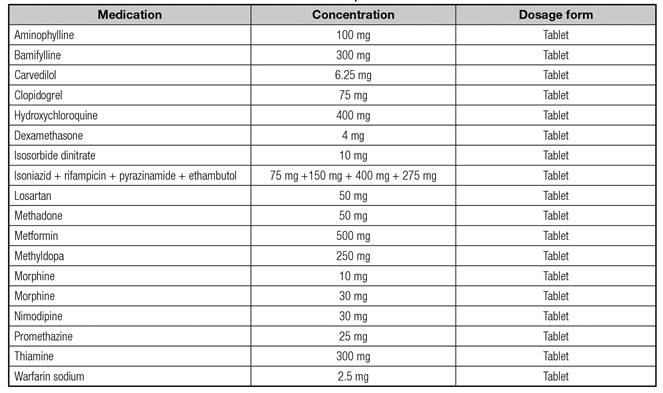
The dosage form and concentration are very important data; therefore, the data presented here cannot be applied to other dosage forms and concentrations since they have not undergone testing for dispersion. A practical example is that, according to our study, both morfin 10 mg and morfin 30 mg tablets must be crushed in a mortar with a pestle for further dispersion, whereas in the case of warfarin sodium 2.5 mg and 5 mg tablets, only the 2.5 mg tablets need to be crushed.
A total of 14.8% (n = 16) of the tested items were successfully dispersed after crushing. Crushing is recommended for the preparation of these medications prior to administration via EFTs and should follow the steps described in table II.
The list of medications that were successfully dispersed after being crushed in a mortar and pestle is presented in table III.
STUDY ON THE SAFETY OF THE USE OF ORAL SYRINGES
Table IV shows the safety analysis of the use of dispensers or syringes for administration of tablets via EFTs and the testing of their connections to intravenous infusion sets and feeding tubes.
Table IV. Results of the testing of the 10 mL parenteral syringe and 10 mL oral syringe connections to intravenous
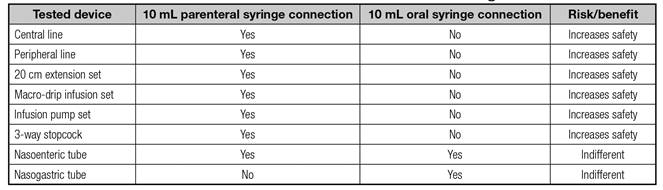
Regarding graduation, the 10-mL syringe has a pair-numbered graduation of 0.2 mL and a Luer lock tip that perfectly fits intravenous needles and infusion sets. The 10-mL dispenser has a 0.2 mL graduation and all mL graduation marks are printed on it; it has a Luer slip tip that connects better to feeding tubes.
The diameter of the dispenser and syringe tips are 3 and 2 mm, respectively. The dispenser has a 50% larger tip that does not fit intravenous devices (Figs. 1 and 2); therefore, its use safely avoids accidental intravenous administration.
DISCUSSION
Solid dosage forms, such as tablets, capsules, and dragees, are administered to hospitalized patients through EFTs. In the study by Heydrich 14, solid dosage forms were used more frequently than liquid forms, indicating that, in clinical practice, patients with EFTs generally do not receive medications as indicated by the data in the literature regarding drug administration, inconveniencing both the patient and the health professional involved in the treatment.
In our study, circa 90% of the oral solid medications tested for administration via EFT presented a "satisfactory" dispersion time or could be crushed for administration via EFTs; however, it is important to emphasize that it is necessary to standardize the administration technique. To promote a standardized procedure, the volumes of water used to disperse such drugs for administration via EFTs were recorded, allowing nursing staff to work in a standardized way to minimize individual errors. Further, in our study, only the drugs that had no alternative oral liquid forms were tested, i.e., those whose dosage forms could not be changed by pharmaceutical intervention.
According to Heineck, Bueno, and Heydrich 13, 95% of the prescribed drugs were solid preparations: tablets (71.9%), capsules (12.2%), coated tablets (9.5%), soluble tablets (2.2%), powders (0.8%) and granules (0.2%). These authors also showed that 23% of the prescribed drugs were solid preparations whose prescription of liquid dosage forms was possible; however, only 5% of the drugs were administered in their liquid forms. Triki et al. 2 reported that it is possible to reduce the risk of administration errors and facilitate drug administration via EFTs through the prescription of oral liquid dosage forms or dispersible oral solid dosage forms. Other studies mentioned that another way to contribute to safer drug administration via EFT is through cooperation with the pharmacist in order to adapt dosage forms for administration more accurately and retain the administration protocol 2,5,16,17.
Our research so far has not studied the content and effectiveness of the tested drugs; it was verified that the administration of medications via EFT is a common, but unregulated, practice in the hospital. It is worth noting that this practice should be suggested when there are no other suitable alternative treatments because solid dosage forms need to be transformed into liquid forms prior to administration via EFTs. Therefore, standardization and practical procedures are required for the implementation of extemporaneous formulations in order to minimize errors in the administration of solid medications via EFTs.
Dashti-Khavidaki et al. 16 showed that clinical pharmacists' education programs significantly improved nurses' knowledge about medication administration via EFTs. Renovato, Carvalho, and Rocha 18 observed, however, that among the nursing professionals interviewed, 86.96% did not undergo a refresher course on pharmacology and medication administration. In view of this alarming number, continuing education concerning this subject is important to reduce errors in the preparation and administration of medications, which can compromise both nutritional support and the effectiveness of drug therapy 18.
Assessing medication dispersion time is crucial to avoid significant reductions in drug efficacy, reduce the risk of contamination from exposure, and avoid taking more of the nursing staff's time than necessary. Complete dispersion of the medication should not take much time, which is why our study established a period of 20 minutes or less for satisfactory dispersion.
When multiple medications are to be administered at the same time, each should be given separately and the feeding tube should be flushed between each medication. Non-compliance with these recommendations may result in future EFT complications, since the mixture of drugs, both in a disposable cup and in the EFT itself, enables interactions 10,17.
The study by Lohmann et al. 15 presents the development and validation of an algorithm to facilitate drug prescription for inpatients with EFTs. The authors found that 83.5% of the tested drugs could be switched to suitable drugs for administration via EFTs; in our study, we found that 90% of the tested oral solid drugs could be adapted for administration via EFTs.
In the study by Heineck, Bueno, and Heydrich 13, omeprazole was one of the most common drugs given to patients. This drug is available in capsules or as soluble tablets; capsules were used in 69.5% of the patients and soluble tablets in 30.5%, even though soluble tablets are more appropriate for EFT cases and were available in the hospital where the research took place. Similarly, paracetamol tablets and codeine and ranitidine-coated tablets also had alternative oral dosage forms and yet the tablet preparations were used in 96.4% and 97.1% of the patients, respectively, while the liquid preparations were only used in 2.9% of the cases where patients had EFTs.
It is important to emphasize that clinical evaluation is sovereign, and it is up to the multidisciplinary team to assess the risk-benefit ratio of the administration of the items contraindicated for administration via EFTs and all the other medications. Thus, as with all interventions in patients that use EFTs, these interventions could not be performed without accounting for the unique medical history of each patient. Taking this into consideration, in the study by Do Nascimento et al. 10, the interventions were made only after an evaluation of their clinical relevance (30.2% of the total potential interventions).
Another aspect that should be considered is the possible changes in dispersion times due to different drug manufacturers. At the hospital where our study took place, the same brands of drugs are not always available for a particular specification since it is a public hospital that buys its medicines through bidding. Differences in formulations are also expected, although they have been minimized by the requirement of bioequivalence for generic or similar pharmaceutical products established by the Collegiate Board of Directors of the Brazilian Health Surveillance Agency, Resolution No. 134, on May 29, 2003 19.
The existing literature has shown that the inadequate administration of medications via the intravenous route can expose patients to increased risks of morbidity and mortality worldwide 9,20,21. The use of the dispenser for administering oral medication via EFTs reduces the risk of giving medications intravenously because its tip does not fit the intravenous devices, as shown by the results of our study concerning the characteristics of the instrument. The dispenser tip has a larger diameter and reduces the need for crushing solid dosage forms for administration via EFTs since small fragments will not occlude the tip. It is important to highlight that medication errors concerning the administration of incomplete doses are the responsibility of everyone involved in the process, from the dispensing to the infusion; therefore, using adequate equipment to avoid incomplete doses can help to minimize the problem 6,9.
CONCLUSIONS
It is possible to standardize the technique for administration of solid dosage forms by dispersing them in 10 mL of water in the dispenser since most studied dosage forms dispersed in a "satisfactory" way. Promoting the use of the dispenser at the expense of the syringe is of utmost importance, as this simple change in behavior can prevent serious incidents with the administration of the dispersion of solid dosage forms via intravenous devices.
Thus, the administration of medications via EFTs in hospitalized patients requires a substantiated and standardized practice that should involve the analysis of the dispersion of these medications and the safe use of dispensers in order to enable the benefits of the proposed pharmacotherapy and the patient's improvement.