INTRODUCTION
Allergic disease is recognized by the World Health Organization (WHO) as one of the four major noninfectious diseases for targeted prevention in the 21st century, and it annually affects 10% to 30% of global population and is growing an important public health concern 1,2. Allergen is one of the causative factors for development of allergic disorders. Particularly, allergens of mite sources are widely recognized as a primary initiator to induce hypersensitive reaction 3-6. As an aspiration allergen widely extant in nature, mite allergen has long received special attention for its universality and particularity 7-10. Therefore, it's of great significance to understand the diverse habitats and breeding materials of acaroid mites in the prevention and treatment of allergic asthma. During November 2009 and 2011, we undertook an investigation on breeding status of acaroid mites in 47 species of the stored rhizomatic traditional Chinese medicinal materials collected from 47 stores and warehouses for traditional Chinese medicinal herbs throughout 17 cities in Anhui province. The present study was aimed at reporting our findings on the mite breeding in the total 47 samples detected.
MATERIALS AND METHODS
SAMPLE COLLECTION
Herbal samples were collected from traditional Chinese medicine store and warehouses in compliance with the breeding habits of acaroid mites, ecological instruments were used to obtain relevant information. The samples were primarily included Radix rehmanniae, Radix puerariae, Bulbus lilii, Radix angelicae sinensis, Radix salviae miltiorrhizae, etc. Apart from that, dusts in the investigated places were also sampled. All of the stored rhizomatic traditional Chinese medicinal materials were stored over 6 months on average. And 10 aliquots of the samples were obtained from each of the stored rhizomatic traditional Chinese medicinal materials, separately sealed in sampling bag and transported to the laboratory, where each sample was measured with the balance by 10 g for each. Sieve shaker was used to separate the dusts from physical samples before final isolation of the acaroid mites.
SEPARATION AND CLASSIFICATION OF ACAROID MITES
Mites in the physical samples were isolated using Tullgren funnel and directicopy, while those in the dusts were extracted with waternacopy and redricopy 11. The mite slides were prepared as previous description from the specimens isolated to undergo light microscopic observation of the morphology and species identification as well as count. Classification of the acaroid mites was in compliance with the taxonomic system described by Hughes 12,13.
INFORMATION ANALYSIS
The number of acaroids mites in different samples of stored material was counted, and the breeding density of acaroid mites was calculated in accordance with the formula (D = N/T × 100%) (N represents the number of acaroid mites; T, the sample quality; and D, the breeding density of acaroid mites). Richness index of species was shown in Margalef index by formula Rmargalef = (S-1)/lnN (where S stands for the number of species; N, total number of every individual species). Diversity index of species was denoted by Shannon-Wiener index in formula H' =- ΣPilnPi) (Pi = Ni/N, or the proportion of individuals belonging to the ith species). Evenness index of species was represented by Pielou's evenness as formula J = H'/Hmax (Hmax = lnS).
RESULTS
SPECIES AND DENSITY OF ACAROID MITES IN DIFFERENT STORED RHIZOMATIC TRADITIONAL CHINESE MEDICINAL MATERIALS
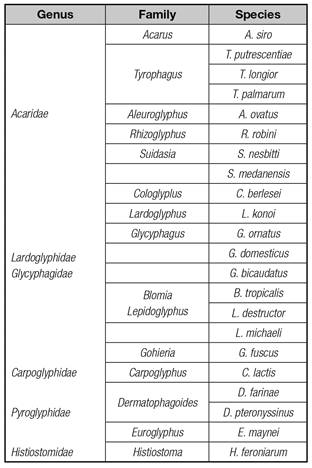
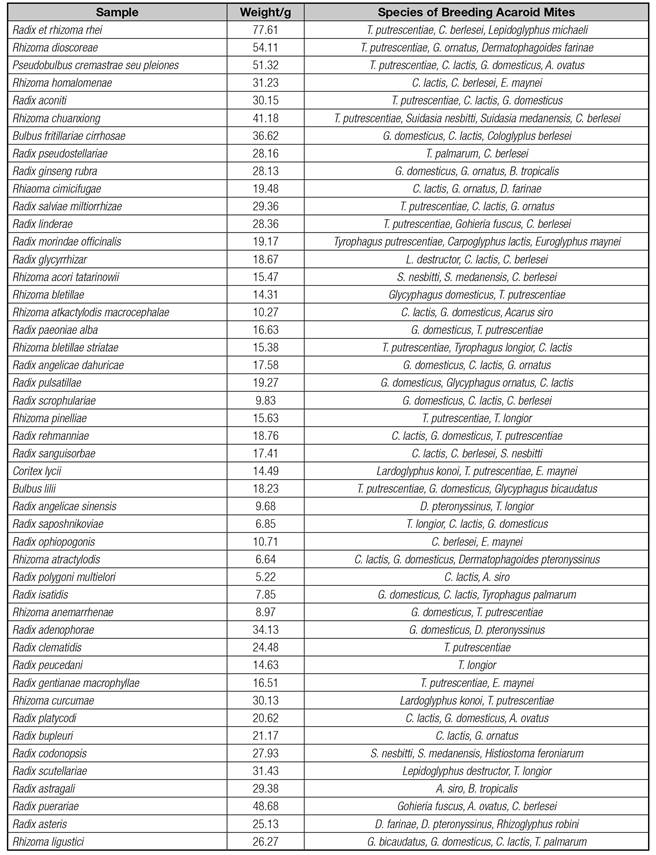
Species and density of acaroid mites in 47 sorts of stored traditional Chinese medicinal materials of root-stock origins are shown in table I, which demonstrates that different species of acaroids mites differ in ecological habits and habitats. Different species of acaroid mites varied to a certain degree in their habitats, feedings and ecological habits as well as in the stored rhizomatic traditional Chinese medicinal materials by breeding densities. A total of 20 species of acaroid mites were separated from 47 kinds of rhizomatic traditional Chinese medicinal materials, belonging to 15 genus and 5 families of the acaridae respectively (Table II). The results indicate a diversity of the acaroid mites in the stored medicinal materials in Anhui area.
Table I Breeding density of acaroid mites in the stored rhizomatic traditional Chinese medicinal materials
ECOLOGICAL PARAMETERS FOR ACAROID MITES IN THE STORED RHIZOMATIC TRADITIONAL CHINESE MEDICINAL MATERIALS
The top five rhizomatic traditional Chinese medicinal materials in the 47 samples with highest breeding density of acaroid mites were involved sequentially in Radix et rhizoma rhei, Rhizoma dioscoreae, Pseudobulbus cremastrae seu pleiones, Radix puerariae, and Rhizoma chuanxiong. The breeding density, number of species, richness index, diversity index, and evenness index are shown in table III, demonstrating diverse ecological habits and habitats for different species of acaroids mites. The highest breeding density was found in Radix et rhizoma rhei, while the highest richness index, diversity index and evenness were seen Pseudobulbus cremastrae seu pleiones. These findings suggested serious contamination of the rhizomatic traditional Chinese medicinal materials withacaroid mites by diverse species and a relatively stable species class.
SEASONAL CHANGES OF ACAROID MITES IN THE STORED RHIZOMATIC TRADITIONAL CHINESE MEDICINAL MATERIALS
In order to examine the community fluctuation of acaroid mites in the rhizomatic traditional Chinese medicinal materials in different month, we performed analysis on the average breeding density, diversity, richness index and evenness index in the samples collected in April, January, July and October, respectively, and found highest breeding species richness index and diversity index in July. Yet the average breeding density was highest in October, and maximal uniform index, in April (Table IV).
SEASONAL CHANGES FOR THE 47 SPECIES ACAROID MITES IN THE STORED RHIZOMATIC TRADITIONAL CHINESE MEDICINAL MATERIALS
By the previous results, we included Radix et rhizoma rhei, Rhizoma dioscoreae and Pseudobulbus cremastrae seu pleiones for further investigation through cultivation under artificial environment. The observation showed that T. putrescentia, C. berlesei and other species of acaroid mites were increased to 53%, 36% and 11%, respectively, after 2-week of cultivation, and the number of T. putrescentia climbed to 15.71 individuals/g at week 4 from 12.47 individuals/g at week 2 and peaked at week 8 by 23.63 individuals/g. By 12th week, the number was decreased to 19.54 individuals/g. Cthe number of C. berlesei was declined to 5.71 individuals/g at week 8 and zero at week 12 from 8.54 individuals/g at 2nd week.
DISCUSSION
House dust mites and stored product mites are ubiquitous and wide in species, and in category of Acarida, Oribatida, Aetinedida and Gamasida. Mites belonging to Acarida include 7 families, namely, Acaridae, Lardoglyphidae, Glycyphagidae, Chortoglyphidae, Carpoglyphidae, Histiostomidae, and Pyroglyphidae 14-18. Acaroid mite is a tiny arthropod widely distributed around the world, most of which live on themselves, feed on organic orts of animals or plants. Their ideal habitats includes grains, Chinese medicinal materials, dry fruits and vegetables in storages, as well as textile fabric and dust in human dwellings 18,19,20. Distribution and density of this species are commonly affected by rainfall and seasonal change. Emergence of it may be quantity in temperature of 15 °C to 16 °C, particularly in summer and autumn with an average temperature of 29-33 °C and humidity of around 68%-76%. Chao-pin LI 21 once investigate on the composition and diversity of stored acaroid mites in Anhui province, and found that the average breeding density, species richness and diversity in southern Anhui areas (including Wuhu area) were higher than those of Huaibei Plain, Jianghuai hilly regions, and plain areas in central Anhui.
In order to understand the acaroid mites in rhizomatic traditional Chinese medicinal materials as well as the growth and decline of this species community in different months, such as indexes on the average breeding density, diversity, , richness and evenness in the same storage, we respectively examined the indexes in January, April, July and October, and found that the highest richness index and diversity index were present in July, while the highest average breeding density was in October, and the maximal evenness index in April. Previous studies described that the distribution of acaroid mites was influenced by humity, temperature, illumination, eating habits of the mites, human interference and other factors 22-24, which were identical to our results that the acaroid mites bred in large quantity in the storages, because of the average relative humidity being 75.6% and mean temperature being 31 °C in July. Besides, the storages sampled were in closure for long time without excellent ventilation and air exchange as well as planned cleaning. Although sampling in October, when the temperature and humidity remained at 25 °C and 66%, demonstrated relative decline of richness and diversity of the acaroid mites, yet the density was maximal. This may be associated with long breeding season, for the mites don't breed until the autumn after female acaroid mites lay their eggs in summer. Even if lower temperature in Spring doesn't favor to the breeding of acaroid mites, those with lower need of temperature and humidity , such as T. putrescentiae, can still easily live and breed. This is why our results demonstrated the highest evenness of the mites in April. As discussed above, we concluded that the average breeding density, diversity index, and richness index of the community as well as evenness index are closely related to humiture without considering the human and food factors, since higher indexes of average density, evenness and diversity were observed in samples collected in summer and autumn seasons. Whereas the acaroid mites would exist in hypopus when the temperature and humidity are unfavorable to its breeding 25.
Relatively higher average breeding density of acaroid mites was found in Radix et rhizoma rhei, Rhizoma dioscoreae, Pseudobulbus cremastrae seu pleiones, Radix puerariae, and Rhizoma chuanxiong, in which T. putrescentiae and C. berlesei are predominant. In order to understand change patterns, particularly the living habits and living environment for T. putrescentiae and C. berlesei, we cultivated Radix et rhizoma rhei, Rhizoma dioscoreae and Pseudobulbus cremastrae seu pleiones in the laboratory by artificially setting the temperature at 20 °C and humidity at 76%. Sampling by every other two weeks showed that the number of C. berlesei were decreasing, while T. putrescentiae continued to breed and peaked at 8th week when the number of species was in saturated state. Subsequently the number of T. putrescentiae was declined somewhat at week 10 and week 12 as a result of emerging of other species that led to insufficient food supply in the same community. These changes are involved in the living habits of acaroid mites and interspecies predominance, because only one predominant species of mites can survive in the same community, and distinctly affect the community population and environment. Besides, higher quantity of emerged mites would lead to higher biomass and stronger survival in the stored materials, eventually resulting in restraining other species of mites from breeding 26,27,28. However, as Cheyletus eruditus, the predator of acacoid mites breeding to a certain quantity emerges, the community will be dynamically balanced 29,30.
Our work suggests that acaroid mites breed extensively in stored rhizomatic traditional Chinese medicinal materials in Anhui province, and their quantity is large in Radix et rhizoma rhei, Rhizoma dioscoreae, Pseudobulbus cremastrae seu pleiones and Radix puerariae. G. domesticus and T. putrescentiae were detected in the traditional Chinese medicinal materials of root-stock origins. Higher richness index, diversity index and breeding density of acaroid mites seen in July showed that breeding of this species are affected by humiture. We observed the growth and decline of C. berlesei and T. putrescentiae under artificial circumstances, and found a sharp decline in the number of C. berlesei, while the number of T. putrescentiae tended to grow before decrease. Although this tendency may be associated with changes in the local environment and climate, yet it remains further investigation.
The species of acaroid mites in the samples may be over our real recordings, because we exclusively identified part of the samples and left other species uncounted. Besides, it is hard to completely isolate entire mites from the samples. The breeding density of sampled mites was represented by the number of mites in the overall samples, and calculated indirectly in proportional samples, for which only mirrors the gross breeding density in various samples. Nevertheless, previous studies indicated that acaroid mites would move and spread around when their breeding density increases to a certain extent in stored rhizomatic traditional Chinese medicinal materials. Migration of the mites tends to spread various microorganisms like bacterium and fungus. Therefore, the dust and the herbal residues in storages should be well deposited in order to prevent transmission of acaroid mites.
In conclusion, we conducted a preliminary investigation on the species, density and diversity of acaroid mites breeding in the stored rhizomatic traditional Chinese medicinal materials. These findings may be additional data for systematic research on the mites in stored products, and supply theoretical evidences for prevention and control of the acaroid mite contamination in the stored rhizomatic traditional Chinese medicinal materials.