Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.34 no.4 Madrid jul./ago. 2017
https://dx.doi.org/10.20960/nh.817
TRABAJO ORIGINAL / Paciente crítico
Glutaminemia prognostic significance in critical surgical patients - An analysis of plasma aminogram profile
Significado pronóstico de la glutaminemia en pacientes quirúrgicos críticos. Análisis del perfil del aminograma plasmático
Beatriz P. Costa1,2*, Paulo Martins2,3*, Carla Veríssimo2,4, Marta Simões2,4, Marisa Tomé1, Manuela Grazina2,4, Jorge Pimentel2,3 and Francisco Castro-Sousa1,2
1"A" Surgical Department. Hospitais da Universidade de Coimbra. Centro Hospitalar e Universitário de Coimbra. Coimbra, Portugal.
2Faculty of Medicine. University of Coimbra. Coimbra, Portugal.
3Intensive Medicine Department. Hospitais da Universidade de Coimbra. Centro Hospitalar e Universitário de Coimbra. Coimbra, Portugal.
4Genetic Biochemistry Department. Center for Neurosciences and Cellular Biology of Coimbra University. University of Coimbra. Coimbra, Portugal
*These authors contributed equally to the present paper.
ABSTRACT
Background: Glutamine depletion is common in the critically-ill patients. Glutaminemia lower than 420 μmol/l has been considered as an independent predictive factor of mortality, but the indications for exogenous glutamine supplementation remain controversial. This study intends to determine the glutaminemia profile in critical surgical patients and to investigate its correlation with the severity indexes and the prognosis.
Methods: A prospective study of 28 adult critical surgical patients was performed. Plasma amino acid concentrations were quantified, by ion exchange chromatography, at the moment of admission and at the first and third days, and compared with those of 11 reference healthy individuals. Severity indexes and parameters of prognosis were registered.
Results: In critical surgical patients, mean glutaminemia at admission was lower than that of control individuals (385.1 ± 123.1 versus 515 ± 57.9 μmol/l, p = 0.002) and decreased until the third day (p = 0.042). Prevalence of severe hypoglutaminemia (< 420 μmol/l) at admission was 64.3%. Baseline glutaminemia correlated with the Simplified Acute Physiology Score II (SAPS II score) (Pearson's correlation coefficient r = -39.4%, p = 0.042), and it was lower in cases of erythrocytes transfusion (339.9 ± 78.8 versus 454.9 ± 148.8 μmol/l, p = 0.013). Glutaminemia at the third day correlated with the duration of invasive ventilation support (r = -65%, p = 0.012) and ICU stay (r = -66.5%, p = 0.009). Glutaminemia below 320 μmol/l, observed in 25% of the patients, was associated with higher in-hospital mortality (42.9 versus 19%, statistically not significant [n.s.]) and lower actuarial survival (212.1 ± 77.9 versus 262.3 ± 32.4 days, n.s.).
Conclusions: Those results underscore the relevance of hypoglutaminemia as an adverse predictive factor in the critical surgical patients. Determination of glutaminemia may contribute to a better definition of the indications for glutamine supplementation.
Key words: Glutaminemia. Plasma aminogram. Critical patients. Surgery. Prognosis.
RESUMEN
Introducción: la hipoglutaminemia es común en los pacientes críticos, pero las indicaciones para la suplementación con glutamina exógena siguen siendo controvertidas. Este estudio pretende determinar el perfil de glutaminemia en pacientes quirúrgicos críticos e investigar su correlación con los índices de gravedad y el pronóstico.
Métodos: se realizó un estudio prospectivo de 28 pacientes quirúrgicos críticos adultos. Las aminoacidemias se cuantificaron mediante cromatografía de intercambio iónico en el momento del ingreso y en el primer y tercer día, y se compararon con las de 11 individuos sanos. Se registraron índices de gravedad y de pronóstico.
Resultados: en pacientes quirúrgicos críticos, la glutaminemia media en el ingreso fue inferior a la de los controles (385,1 ± 123,1 versus 515 ± 57,9 μmol/l, p = 0,002) y disminuyó hasta el tercer día (p = 0,042). La prevalencia de hipoglutaminemia severa (< 420 μmol/l) en el ingreso fue de 64,3%. La glutaminemia basal se correlacionó con el SAPS II (r = -39,4%, p = 0,042), y fue menor en los casos de transfusión de eritrocitos (339,9 ± 78,8 versus 454,9 ± 148,8 μmol/l, p = 0,013). La glutamina al tercer día se correlacionó con la duración de la ventilación invasiva (r = -65%, p = 0,012) y de la estancia en la UCI (r = -66,5%, p = 0,009). La glutaminemia < 320 μmol/l, observada en el 25% de los pacientes, se asoció con mayor mortalidad hospitalaria (42,9 versus 19%, n.s.) y menor supervivencia actuarial (212,1 ± 77,9 versus 262,3 ± 32,4 días, n.s.).
Conclusiones: estos resultados refuerzan la importancia de hipoglutaminemia como un factor predictivo adverso en los pacientes quirúrgicos críticos. La determinación de glutaminemia puede contribuir a una mejor definición de las indicaciones para la suplementación.
Palabras clave: Glutaminemia. Plasma aminograma. Pacientes críticos. Cirugía. Prognosis.
Introduction
Glutamine is the most abundant non-essential amino acid in the human body and it is considered as a conditionally essential amino acid in stress conditions (1).
Glutamine depletion, reflected by a plasma glutamine concentration below 420 µmol/l, has been considered to be an independent predictive factor of mortality in critically ill patients (2,3).
Nevertheless, exogenous glutamine supplementation in this context remains highly controversial (4-7). Most described outcome benefits of glutamine supplementation are based on older, smaller and mainly single-center studies involving intravenous administration (8). In the latest meta-analysis published by the Cochrane Database of Systematic Reviews (9), mortality reduction was not verified and only moderate- and low-level evidence on reduction of morbidity was found (infection rate and days on mechanical ventilation, and length of hospital stay, respectively), with high risk of overall bias, suspected publication bias and moderate to substantial heterogeneity within the included studies. Nevertheless, in a meta-analysis of 15 randomized controlled trials including 2,862 patients, Chen QH et al. (10) documented a reduction of the nosocomial infections rate with glutamine supplementation in surgical ICU patients. Moreover, in a systematic review of 26 studies involving 2,484 patients, Wischmeyer PE et al. (1) observed that parenteral glutamine supplementation of nutrition support in critical illness was associated with a significant reduction in hospital mortality and in hospital length of stay, a strong trend towards a reduction in infectious complications and ICU length of stay, and a trend towards reduction of overall mortality. More recently, Van Zanten ARH et al. (8) verified, in a systematic review and meta-analysis of 11 studies including 1,079 adult critically ill patients, that enteral glutamine supplementation was associated with a significant decrease in hospital length of stay (with no effect on hospital mortality, infections complications or length of stay in ICU), and a significant reduction in hospital mortality in burned patients. However, two of the most recent large multicenter randomized controlled studies, the Redoxs and MetaPlus trials, suggested that glutamine supplementation was associated with increased long-term mortality (particularly in medical patients) (11,12).
Consequently, despite controversial glutamine administration protocols in some studies (4-7), safety and efficacy concerns emerged that challenged previous recommendations. The recently published American Society for Parenteral and Enteral Nutrition/Society of Critical Care Medicine guidelines (13) recommended that parenteral glutamine supplementation should not be used routinely in the adult critical care setting, and suggest that supplemental enteral glutamine should not be routinely added to the enteral nutrition regimen, according to the available quality of evidence.
It may be hypothesized that severe hypoglutaminemia in surgical critically ill patients may be associated with adverse outcomes and, therefore, the determination of glutamine plasma levels in high risk conditions may allow a better definition of the indications for exogenous supplementation.
Present research intends to determine the longitudinal profile of plasma concentration of glutamine in surgical critically ill patients in the first three days after admission, to correlate the glutaminemia with the plasma concentrations of other amino-acids and to investigate its correlation with the severity indexes and the prognosis.
Material and methods
A prospective single-center observational study of adult surgical critically ill patients urgently admitted in the Intensive Care Unit (ICU) of Centro Hospitalar e Universitário de Coimbra, Coimbra (Portugal), was implemented between October 2013 and April 2014. Eligible patients were adult patients aged at least 18 years-old, non-electively admitted to the ICU, with surgical pathology, in consonance with the critical illness definition of the Intensive Care Society (14) and with an ICU hospitalization predictably higher or equal to three days. Rejection criteria included pregnancy, lactation, renal insufficiency (creatininemia ≥ 2 mg/dl), acute liver failure (defined in agreement with formerly described criteria [15,16]) and amino acid metabolism diseases.
Study was authorized by the institution's Ethics Committee and followed the principles of the Declaration of Helsinki (17). All subjects (or their representatives) were informed of the nature and purpose of the investigation and gave their consent to participate.
Patients' age and gender were collected. Type of admittance was defined as primary or non-primary (after previous care on other hospital or department). Severity indexes were recorded at the time of entrance, including Acute Physiology and Chronic Health Evaluation II (APACHE II) score (18), Simplified Acute Physiology Score II (SAPS II) (19), Sequential Organ Failure Assessment (SOFA) score (20) and Shock Index (21). Comorbidities were characterized as stated in the Charlson's index (22). Mechanical ventilation, erythrocytes transfusion, amines infusion, renal substitution therapy, surgical interventions and nutritional support were listed, as well as glutamine exogenous supplementation. Enteral nutrition was implemented with standard regimens and provided 25 kcal/kg/day and 1.2 g of proteins/kg/day in patients with body mass index between 18 and 30 kg/m2 (ideal body weight was considered in the remaining cases). Intravenous glutamine was administered in patients on parenteral nutrition, at the dose of 0.2-0.4 mg/kg/day, as a continuous infusion for 24 hours every day.
Evaluation was completed at the time of admittance in the ICU, at the first and the third days, with quantification of plasma amino acid levels (arginine, citrulline, ornithine, glutamine, alanine, proline, glutamic acid, leucine and isoleucine) and routine laboratory tests, including blood gases analysis, lactacidemia, serum biochemistry with creatinine, albumin, aspartate aminotransferase, alanine transaminase, gamma-glutamyl transpeptidase, alkaline phosphatase, bilirrubinemia, lactate dehydrogenase, creatine phosphokinase and C-reactive protein; hemograme and prothombinemia.
Plasma concentrations of amino acids were measured by ion exchange chromatography in a high pressure system (Biochrom 30 analyzer). Plasma was retrieved from blood collected in ethilenediaminotetraacetic acid, by centrifugation at 4,000 g, during ten minutes, and reserved at 4 oC. Samples were prepared with 12% ditiotreitol, five to ten minutes, deproteinized with sulfosalicilic acid, 60 minutes at room temperature and, after separation of the sediment by centrifugation, were filtered and preserved at -20 oC for posterior analysis.
Plasma amino acid concentrations of surgical critical patients were compared with those of a previous historical reference group of eleven fasted healthy subjects (23).
Primary goals included in-hospital mortality rate and actuarial survival. Secondary targets were health care-associated infections rate (24), duration of mechanical ventilation support, ICU and hospital extent of stay, multiple organ failure (MOF) and multiple organ dysfunction (MOD) at the sixth day of the ICU stay, prolonged MOD, relative variation of SOFA score between the admission and the sixth day (∆SOFA6) and performance status at the last examination (Karnofsky index [25]). Health care-associated infections were considered conforming to the criteria of the National Healthcare Safety Network (NSHN), Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA (24). MOF was defined as failure (organ score SOFA ≥ 3 points) of at least two of the six listed organs or systems of the SOFA, including respiratory, cardiovascular, renal, hepatic, neurological and hematological (26). MOD was considered when SOFA score was at least six points and prolonged MOD, as MOD lasting over seven days (27). Relative variation of SOFA between the admission and the sixth day, designated by ∆SOFA6, was calculated in accordance to the formula: ∆SOFA6 = (SOFA score at the sixth day/admission SOFA score)×100-100.
Statistical analysis was undertaken with SPSS Software version 18.0 for Windows (SPSS Inc., Chicago, IL), using Chi-squared, Student's t, paired Student's t, Kaplan Meier and log rank tests, Pearson's correlations and receiver operating characteristic (ROC) curves. Level of significance was considered p < 0.05. Data were expressed as n (%) or mean ± standard deviation (SD).
Results
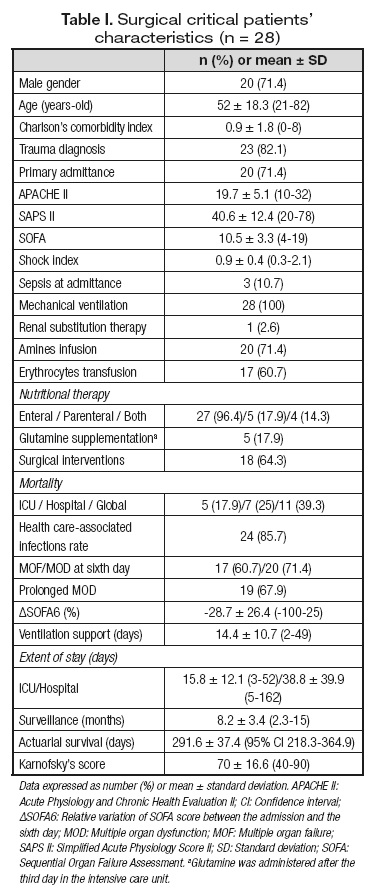
Surgical critically ill patients (n = 28) were included with the characteristics summarized in table I.
Trauma was the most frequent diagnosis (82.1%); other entities included severe acute pancreatitis, ischemic colitis, cholangitis associated with main bile duct lithiasis, primary bacterial peritonitis and abdominal aorta aneurism rupture (n = 1, respectively).
All patients performed the determination of plasma aminogram at the ICU ingress, 21 fulfilled both the admittance and the first day evaluations, and 14 completed all the three moments of evaluation.
Mean age and gender distribution of eligible ICU patients were similar to the control subjects (52.1 ± 18.3 vs 46.3 ± 15.1 years-old, n.s., and 71.4 vs 54.5% of male gender, n.s., respectively).
At the admission in the ICU, in comparison with the reference subjects, critical surgical patients demonstrated lower mean plasma concentrations of glutamine, citrulline and ornithine and higher mean plasma levels of glutamic acid (Fig. 1). In those patients, mean plasma concentrations of citrulline, arginine, ornithine and glutamic acid increased until the third day, while glutaminemia decreased.
In fact, in surgical critically ill patients, mean glutaminemia at the admission was lower than that of control individuals (385.1 ± 123.1 [205.1-776.5] versus 515 ± 57.9 [424-651] μmol/l, p = 0.002] and decreased until the third day (385.1 ± 123.1 [205.1-776.5] versus 288.8 ± 110.5 [23.9-458.6] μmol/l, p = 0.042) (Fig. 1). Severe hypoglutaminemia prevalence was high: 64.3% of surgical critical patients demonstrated a glutaminemia at admission lower than 420 μmol/l, and 25% revealed a concentration inferior to 320 μmol/l.
At the ingress in the ICU, glutamine accounted 50.7 ± 14.9% of the studied amino acids plasma concentration; that percentage decreased until the first day (50.7 ± 14.9% versus 46.2 ± 14.1%, p = 0.004) and between the first and the third day (46.2 ± 14.1% versus 35.2 ± 14.4%, p = 0.0001).
Admission glutaminemia correlated significantly with argininemia (Pearson's correlation coefficient r = 76.2%, p =0.0001), alaninemia (r = 74.7%, p = 0.0001), ornithinemia (r = 46.5%, p = 0.013), leucinemia (r = 46.7%, p = 0.012) and isoleucinemia (r = 41.5%, p = 0.028) (Fig. 2A); correlations with the remaining laboratorial parameters were not statistically significant, except with C-reactive protein (r = 65.3%, p = 0.003).
Baseline glutaminemia correlated with SAPS II score (r = -39.4%, p = 0.042) (Fig. 2A and Table II). Severe hypoglutaminemia (under 420 μmol/l) was more frequent in patients with SAPS II score over 32 (71.4 versus 16.7%, p = 0.027; sensitivity = 93.8%, specificity = 45.5%, accuracy = 74.1%; negative predictive value = 83.3%; positive predictive value = 71.4%). Admission glutaminemia was lower in cases of erythrocytes transfusion (339.9 ± 78.8 versus 454.9 ± 148.8 μmol/l, p = 0.013) and in patients with prolonged MOD (345.6 ± 85.4 versus 430.1 ± 114.4 μmol/l, p = 0.051) (Table II). It also correlated significantly with hepatic SOFA score at admittance (r = 38.5%, p = 0.043) and at the third and sixth day (r = 53%, p = 0.004 and r = 50.2%, p = 0.008, respectively).
Glutaminemia at the third day correlated negatively with the duration of invasive ventilation support (r = -65%, p = 0.012) and ICU stay (r = -66.5%, p = 0.009) (Fig. 2B).
Patients with baseline glutaminemia lower than 320 μmol/l were predominantly of female gender (57.1 versus 19%, n.s.); they also had higher mean comorbidity Charlson's index (1.4 ± 1.9 versus 0.7 ± 1.8, n.s.), higher prevalence of non-trauma diagnosis (28.6 versus 14.3%, n.s.) and septic conditions (14.3 versus 9.5%, n.s.), and higher erythrocytes transfusion requirements (71.4 versus 57.1%, n.s.).
Decrease of mean glutaminemia between admission and the third day of ICU stay was higher in hospital non-surviving patients than in surviving individuals (17.3 ± 37.3 versus 3.8 ± 36.8%, n.s.) (Fig. 3A).
Admission glutaminemia below 320 μmol/l, observed in 25% of the patients, was associated with higher prevalence of MOF and MOD at the sixth day (71.4 versus 57.1%, n.s., and 85.7 versus 66.7%, n.s., respectively), prolonged MOD (85.7 versus 68.4%, n.s.), higher ∆SOFA6 (-25.2 ± 37.6 versus -29.9 ± 22.6%, n.s.), higher in-hospital mortality (42.9 versus 19%, n.s.), longer hospital stay (44.3 ± 54.6 versus 37 ± 35.2 days, n.s.) and lower actuarial survival (212.1 ± 77.9 versus 262.3 ± 32.4 days, n.s.) (Fig. 3B).
Outcome parameters of patients submitted to parenteral nutrition were not significantly different from those observed in patients who performed exclusively enteral nutrition.
Discussion
Exogenous glutamine administration is one of the most controversial issues in critical care (4-7).
Studies on glutamine supplementation in critically ill patients have been characterized by the heterogeneity of the studied populations (including severity of illness, associated organ failures such as hepatic and renal dysfunctions, presence of non-resuscitated shock, nutritional status and demographic characteristics, among others), additional co-interventions (such as simultaneous provision of other additives like selenium), different caloric and protein intakes and, occasionally, poor methodological quality. Analysis of the results is also hampered by differences in the glutamine administration schedule: dosage (0.2 to 0.8 g/kg/day), route of delivery (intravenous, enteral or combined), timing (in the acute phase or later), duration and type of provision (nutritional supplementation or pharmacotherapy independent of nutritional support) (1,6,11,12,28,29). Furthermore, in previously published trials, glutamine plasma concentration was not routinely measured at the time of study enrollment (1,7,11,12,28,29).
Severe glutamine depletion in critically ill patients, with plasma levels under 420 µmol/l, has been considered as a pejorative prognostic factor (2,3) and has demonstrated a variable prevalence, described between 25 and 67% (1,12,30,31). In addition, high baseline plasma concentrations of glutamine have been also associated with increased mortality rate. In fact, low (below 420 µmol/l) and high (above 930 µmol/l) glutaminemia within 24 hours of admission in a mixed ICU has been recognized as an independent predictor for six-month mortality and to increase the sensitivity of APACHE II score to predict death (2). Some authors consider glutamine plasma levels above 700 µmol/l as a clear contraindication for glutamine supplementation (5).
In the present study, prevalence of severe hypoglutaminemia in surgical critical patients at the admission in the ICU was high and mean glutaminemia values were similar to those described by Van Zanten et al. (12). A significant moderate positive correlation was demonstrated between admission glutaminemia and severity score SAPS II, contrary to the previous studies (2). A SAPS II score higher than 32 revealed to be a predictive factor of severe hypoglutaminemia with high sensitivity and negative predictive values. Positive correlations between glutaminemia at admission and hepatic SOFA scores at admission and at the third and sixth days were in concordance with the association between liver dysfunction and high plasma glutamine concentrations described in the literature (31). The present findings did not corroborate the inverse correlation between admission glutaminemia and C-reactive protein levels observed by Nienaber A et al. (32) in a mixed ICU patients cohort.
In this series, glutamine depletion was aggravated during the first three days of ICU stay and glutaminemia at the third day demonstrated a significant and high negative correlation with the duration of invasive ventilation support and the ICU length of stay. Severe hypoglutaminemia at admission, below 320 µmol/l, was associated, although not significantly, with higher prevalence of multiple organ failure at the sixth day and in-hospital mortality. Patients with prolonged multiple organ dysfunction demonstrated lower mean baseline glutaminemia levels. Therefore, in this study, hypoglutaminemia represented a pejorative prognostic factor, namely of prolonged ventilation support requirement.
High positive correlations observed between admission glutaminemia and plasma concentrations of alanine and arginine were expected since glutamine and alanine are both depleted in catabolic states (32) and glutamine serves as a precursor for de novo production of arginine through the citrulline-arginine pathway (33,34). A moderate direct correlation was also demonstrated between admission glutaminemia and plasma levels of two essential branched-chain amino acids, leucine and isoleucine. Branched-chain amino acids, especially leucine, stimulate protein synthesis, inhibit proteolysis, and promote glutamine and alanine production; its oxidation in skeletal muscle compensates for the increased energy expenditure and glutamine consumption in catabolic states (32).
Decrease of mean glutaminemia levels until the third day and concomitant increase of plasma concentrations of citrulline, arginine, ornithine and, mainly, of glutamic acid may indicate the favoring of two metabolic pathways: synthesis of citrulline (in the enterocyte), precursor of arginine (35,36), and production of energy (through the supply of energetic substrates for gluconeogenesis) (37).
The main limitations of the present study include the small number of patients and the non-consecutive enrollment. The exclusively surgical cohort was characterized by the predominance of trauma diagnosis, the high mean severity scores and the high rate of health care-associated infections. Despite the higher prevalence of severe hypoglutaminemia in patients with high severity score SAPS II, it was not possible to define the profile of glutamine-depleted patients clearly and, consequently, to select a subgroup who may benefit most from supplementation.
Considering the high variability of glutaminemia at admission in ICU (1,12,30,31), the potential benefits of supplementation on glutamine-depleted patients (1,8-10) and the risks of the glutamine administration in cases with high plasma concentration (2,11), determination of glutamine plasma levels should precede exogenous supplementation.
Nowadays, glutaminemia measurement is not readily available as a routine and fast analysis in most hospitals. Recently, a point-of-care instrument was proposed as a useful bedside screening method in the ICU, although not sufficiently accurate to replace quantitative plasma analysis by standard techniques (39).
Additional knowledge of glutamine kinetics and turnover (including the relation between plasma concentration and availability) and characterization of epidemiology of glutamine depletion in critical care are needed, particularly in surgical patients. Further high quality studies are warranted to determine the safety and cost-effectiveness of glutamine supplementation in the intensive care setting.
Conclusions
Those results underscore the high prevalence of severe hypoglutaminemia in the critical surgical patients and its relevance as a predictive factor of unfavorable outcome.
Determination of glutaminemia may contribute to a better definition of the indications for glutamine supplementation in critically ill surgical patients.
Statement of authorship
All the authors meet all of the authorship criteria, namely substantial contribution to conception and design, and/or acquisition of data, and/or analysis and interpretation of data; drafting or revising the article and approval of the version to be submitted and any revised version to be published.
AUTHORS' CONTRIBUTIONS
BPC and PM: Conception and design of the study; acquisition, analysis and interpretation of data, and writing the article.
CV, MS and MT: Acquisition of data and revision of the article.
MG: Acquisition, analysis and interpretation of data; revision of the article.
FCS and JP: Interpretation of data and revision of the article.
All authors: Reading and approval of the final version of the manuscript.
Declaration
This series was partially presented as a poster presentation at the 37th European Society for Clinical Nutrition and Metabolism (ESPEN) Congress, Lisbon, on September 7th, 2015, and the abstract was published in Clinical Nutrition (Costa BP, Martins P, Veríssimo P, Simões M, Tomé M, Marques G, Grazina M, Pimentel J, Castro Sousa F. Plasma glutamine concentration - A predictive factor of actuarial survival in critical surgical patients? Clin Nutr 2015;34(Supp 1):S230-1).
References
1. Wischmeyer PE, Dhaliwal R, McCall M, Ziegler TR, Daren K, Heyland DK. Parenteral glutamine supplementation in critical illness: A systematic review. Crit Care 2014;18:R76. [ Links ]
2. Rodas PC, Rooyackers O, Hebert C, Norberg A, Wernerman J. Glutamine and glutathione at ICU admission in relation to outcome. Clin Sci (Lond) 2012;122:591-7. [ Links ]
3. Oudemans-van Straaten HM, Bosman RJ, Treskes M, Van der Spoel HJ, Zandstra DF. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med 2001;27:84-90. [ Links ]
4. Ginguay A, De Bandt JP, Cynober L. Indications and contraindications for infusing specific amino acids (leucine, glutamine, arginine, citrulline, and taurine) in critical illness. Curr Opin Clin Nutr Metab Care 2016;19:161-9. [ Links ]
5. Cynober L, De Bandt JP. Glutamine in the intensive care unit. Curr Opin Clin Nutr Metab Care 2014;17:98-104. [ Links ]
6. Van Zanten ARH. Glutamine and antioxidants: Status of their use in critical illness. Curr Opin Clin Nutr Metab Care 2015;18:179-86. [ Links ]
7. Van Zanten ARH, Hofman Z, Heyland DK. Consequences of the REDOXS and METAPLUS trials in the end of an era of glutamine and antioxidant supplementation for critically ill patients. JPEN J Parenter Enteral Nutr 2015;39:890-2. [ Links ]
8. Van Zanten ARH, Dhaliwal R, Garrel D, Heyland DK. Enteral glutamine supplementation in critically ill patients: A systematic review and meta-analysis. Crit Care 2015;19:294. [ Links ]
9. Tao KM, Li XQ, Yang LQ, Yu WF, Lu ZJ, Sun YM, et al. Glutamine supplementation for critically ill adults. Cochrane Database Syst Rev 2014;9:CD010050. DOI: 10.1002/14651858.CD010050.pub2. [ Links ]
10. Chen QH, Yang Y, He HL, Xie JF, Cai SX, Lieu AR, et al. The effect of glutamine therapy on outcomes in critically ill patients: A meta-analysis of randomized controlled trials. Crit Care 2014;18:R8. [ Links ]
11. Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al.; Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489-97. [ Links ]
12. Van Zanten AR, Sztark F, Kaisers UX, Zielmann S, Felbinger TW, Sablotzki AR, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: A randomized clinical trial. JAMA 2014;312:514-24. [ Links ]
13. Taylor, BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al.; Society of Critical Care Medicine and the American Society of Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med 2016;44:390-438. [ Links ]
14. Intensive Care Society. Levels of Critical Care for Adult Patients - Intensive Care Society. 2009. Available at: https://www2.rcn.org.uk/__data/assets/pdf_file/0005/435587/ICS_Levels_of_Critical_Care_for_Adult_Patients_2009.pdf. Accessed 24 Aug 2016. [ Links ]
15. O'Grady JG, Schalm SW, Williams R. Acute liver failure: Redefining the syndromes. Lancet 1993;342:273-5. [ Links ]
16. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al.; CANONIC Study Investigators of the EASL-CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterol 2013;144:1426-37.e1-9. [ Links ]
17. World Medical Association: World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/17c.pdf. 2008. Accessed in September 23, 2015. [ Links ]
18. Fagon JY, Chastre J, Novara A, Medioni P, Gibert C. Characterization of intensive care unit patients using a model based on the presence or absence of organ dysfunctions and/or infection: The ODIN model. Intensive Care Med 1993;19:137-44. [ Links ]
19. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63. [ Links ]
20. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [ Links ]
21. Rady MY, Smithline HA, Blake H, Nowak R, Rivers E. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med 1994;24:685-90. [ Links ]
22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [ Links ]
23. Pinto Costa B, Serôdio M, Simões M, Veríssimo C, Castro Sousa F, Grazina M. Citrullinemia stimulation test in the evaluation of the intestinal function. Nutr Hosp 2013;28:202-10. [ Links ]
24. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. [ Links ]
25. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncol 1984;2:187-93. [ Links ]
26. Fröhlich M, Wafaisade A, Mansuri A, Koenen P, Probst C, Maegele M, et al. Which score should be used for posttraumatic multiple organ failure? - Comparison of the MODS, Denver-and SOFA-Scores. Scand J Trauma Resusc Emerg Med 2016;24:130. [ Links ]
27. Shepherd JM, Cole E, Brohi K. Contemporary patterns of multiple organ dysfunction in trauma. Shock 2017;47(4):429-35. [ Links ]
28. Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, et al.; Scottish Intensive care Glutamine or seleNium Evaluative Trial Trials Group. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011;342:d1542. [ Links ]
29. Wernerman J, Kirketeig T, Andersson B, Berthelson H, Ersson A, Friberg H, et al.; Scandinavian Critical Care Trials Group. Scandinavian glutamine trial: A pragmatic multi-centre randomized clinical trial of intensive care unit patients. Acta Anaesthesiol Scand 2011;55:812-8. [ Links ]
30. Buter H, Bakker AJ, Kingma WP, Koopmans M, Boerma EC. Plasma glutamine levels in patients after non-elective or elective ICU admission: An observational study. BMC Anesthesiol 2016;16:15. [ Links ]
31. Helling G, Wahlin S, Smedberg M, Pettersson L, Tjäder I, Norberg A, et al. Plasma glutamine concentrations in liver failure. PLoS ONE 2016;11:e0150440. [ Links ]
32. Nienaber AP, Dolman RC, Van Graan AE, Blaauw R. Prevalence of glutamine deficiency in ICU patients: A cross-sectional analytical study. Nutr J 2016;15:73-81. [ Links ]
33. Choudry HA, Pan M, Karinch AM, Souba WW. Branched-chain amino acid-enriched nutritional support in surgical and cancer patients. J Nutr 2006;136:314S-8S. [ Links ]
34. Vermeulen MA, Van de Poll MC, Ligthart-Melis GC, Dejong CH, Van den Tol MP, Boelens PG, et al. Specific amino acids in the critically ill patient - Exogenous glutamine/arginine: A common denominator? Crit Care Med 2007;35:S568-76. [ Links ]
35. Cynober L, Moinard C, De Bandt JP. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin Nutr 2010;29:545-51. [ Links ]
36. Marini JC. Interrelationships between glutamine and citrulline metabolism. Curr Opin Clin Nutr Metab Care 2016;19:62-6. [ Links ]
37. Peters JHC, Wierdsma NJ, Teerlink T, Van Leeuwen PAM, Mulder CJJ, Van Bodegraven AA. The citrulline generation test: Proposal for a new enterocyte function test. Aliment Pharmacol Ther 2008;27:1300-10. [ Links ]
38. Xiao D, Zeng L, Yao K, Kong X, Wu G, Yin Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids 2016;48:2067-80. [ Links ]
39. Pettersson L, Rydén S, Smedberg M, Tjäder I, Rooyackers O, Wernerman J. Validation of a point-of-care instrument for bedside glutamine screening in the intensive care unit. Clin Nutr 2015:pii:S0261-5614(15)00262-9. DOI: 10.1016/j.clnu.2015.10.008. [ Links ]
![]() Correspondence:
Correspondence:
Beatriz Pinto da Costa.
"A" Surgical Department.
Clínica Universitária de Cirurgia III.
Hospitais da Universidade de Coimbra
Centro Hospitalar e Universitário de Coimbra.
Praceta Prof. Mota Pinto. 3000-075 Coimbra, Portugal.
e-mail: beatrizpcosta@huc.min-saude.pt
Received: 30/11/2016
Accepted: 15/03/2017