INTRODUCTION
Cirrhosis is defined as the histological development of regenerative nodules surrounded by fibrous bands in response to chronic liver injury, and it can lead to portal hypertension and end-stage liver disease. Although the etiology of cirrhosis is variable, its main causes in developed countries are excessive alcohol consumption and hepatitis C 1. Liver disease is also related to numerous nutritional vitamin and mineral deficiencies 1,2,3,4,5,6 and to the impaired metabolism of carbohydrates, proteins, and lipids. Among minerals, zinc (Zn) and copper (Cu) are essential micronutrients whose role is widely studied for their implication in liver cirrhosis 7.
The liver is involved in the homeostatic regulation of Zn and its levels are decreased in liver cirrhosis since some functions like urinary excretion are altered 8. Some authors have suggested that Zn depletion is a cause of hepatic fibrosis 9. Immune function depression, an increased infection rate, diarrhea, alopecia, and mental disorders are symptoms frequently observed in patients with liver cirrhosis and have been attributed by some authors to Zn deficiency; this also contributes on the predisposition and perpetuation of liver damage 3,4,6,10,11,12,13,14,15,16. It is seems to have an important role in the appearance of liver fibrosis because Zn is the most effective inhibitor for prolyl hydroxylase, an enzyme which plays a key role in collagen synthesis 11. The antioxidant action of Zn may be related to the ability of metalloproteins to remove the excess collagen produced by hepatic stellate cells 11).
The impaired liver function as a result of liver damage in liver cirrhosis leads to lower ability to detoxify substances. The liver regulates homeostasis and Cu distribution to overall system, so prolonged exposure to Cu toxicity contributes to liver cirrhosis, damaging renal tubules and other organs 17. Others studies have found that the Cu reagent generated in a specific process participated directly or indirectly in liver damage produced by stimulation of Kupffer cells, which contributes in the progression of liver fibrosis 3,18,19. In addition, Cu is a cofactor of lysyl oxidase, which participates in collagen formation, while increased concentrations of Cu and its accumulation in the liver have been reported to promote also hepatic fibrosis 3,18.
Liver cirrhosis involves complications such as ascites, hepatorenal syndrome or hepatocellular carcinoma 20, and higher serum Cu/Zn ratios have been observed in patients undergoing hemodialysis 21,22 and in those with hepatocellular carcinoma 23. This ratio could be considered as an interesting tool used in the evaluation of liver cirrhosis in elderly people because Malavolta et al. 24 have described the Cu/Zn ratio as an important inflammatory/nutritional biomarker of all-cause mortality and it also has been correlated with reductions in bone density, physical performance, and overall health status in this group 25. The clinical relevance of the Cu/Zn ratio is considered greater than that of the concentration of each element 26.
The hypothesis of the study is that serum Zn and Cu concentrations as well Cu/Zn ratios impair in cirrhosis, and this impairment would enhance the disease severity measured by the Child-Pugh index score. Few data appear to be available on the relationship between liver disease severity and serum Zn or Cu concentrations or Cu/Zn ratios. Other objectives are to determine serum concentrations of these elements in cirrhotic patients and healthy controls, using flame atomic absorption spectrometry, and to examine the influence on these values of age, sex, death, and its complications.
MATERIALS AND METHODS
The design of the study was previously described in detail elsewhere 27. The initial study population comprised patients with liver cirrhosis under follow-up in the Department of Digestive Diseases of our reference hospital 27. The diagnosis of cirrhosis was based on clinical and ultrasound criteria. Healthy controls were enrolled from among blood donors in the same geographic area. All subjects were fully informed of the aim of the study, and an informed consent was obtained from each one before blood was drawn 27.
This cross-sectional study was conducted in patients with liver cirrhosis in the Department of Digestive Diseases of the Hospital Universitario Virgen de las Nieves in Granada (Southern Spain) and in healthy controls. Exclusion criteria for study participation were: the presence or history of non-insulin-dependent diabetes mellitus, wide bowel resection, gastrectomy, inflammatory bowel disease, cancer, chronic renal failure, active alcoholism, corticoid therapy, or hypercortisolism, the receipt of drugs or dietary supplements known to have antioxidant capacity or interfere with muscle function, and the failure to sign informed consent. The study, including the release of human serum samples, was approved by the Ethics Committee of the hospital and was conducted in accordance with the Declaration of Helsinki. The final study sample comprised 92 patients with liver cirrhosis (38 women and 54 men, with mean ± standard deviation [SD] age of 41.4 ± 16.5 y) and 30 healthy adults from among blood donors at the hospital (13 women and 17 men, with mean age of 40.7 ± 18.8 y).
The patients were divided into two groups according to their Child-Pugh index (CPI) score: 5-6 points = CPI-A (low severity, well-balanced; n = 45); 7-9 points = CPI-B (medium severity, significant functional impairment; n = 47); or 10-15 points = CPI-C (high severity; n = 1; therefore, this patient has been excluded, and this group has not been considered) 28. The CPI score is the sum of points assigned by the physician for degree of ascites, concentrations of plasma bilirubin and albumin, prothrombin time, and degree of encephalopathy. The original CPI was designed to stratify surgical risk in patients with blood imbalance 28 and was subsequently modified 29 by replacing the nutritional status parameter with prothrombin time 30. The present patients were also divided into three age groups (< 30, 31-50, and > 50 y). The present study patients were also divided into eight groups in relation to disease complications: four in the group of complications in the past (ascites, bacteremia-sepsis, varicose vein and encephalopathy), which included patients whose complications were diagnosed and medically followed by the physicians of the Department of Digestive Diseases of the Hospital Universitario Virgen de las Nieves in Granada along one year; and the same four complications in the group with complications at admission in the hospital, which included patients with de novo complications that were diagnosed along the same year when they went in a critical status to the Urgency Service of the hospital.
Blood samples were drawn after overnight fasting from the antecubital vein of each participant while seated. Samples were left to spontaneously coagulate and were then centrifuged at 3,000 g to obtain serum separated by gelose. Aliquots (1 ml) of serum were frozen at -25 °C for transportation and storage in the laboratory of the Department of Nutrition and Food Sciences (University of Granada) until their analysis 31.
Shortly after their delivery, samples were thawed and homogenized for Zn and Cu determination, diluting 200 μl serum in Milli-Q(r) water (1:5) using Eppendorf Tubes(r) and following a previously optimized procedure 31.
All chemicals were analytical reagent grade or higher purity. Bidistilled deionized water with a specific resistivity of 18 MΏ cm-1was obtained using the Milli-Q(r) system (Millipore, Milford, MA). Total Zn and Cu in samples were measured by flame atomic absorption spectrometry using a Perkin-Elmer(r) Model 1100B spectrometer (Perkin-Elmer, Norwalk, CT, USA). The absorbance was correlated with Zn and Cu concentrations by the linear calibration technique. The accuracy and precision of the method were tested on 0148 Serum metal control A level 1 reference material (Kaulson Laboratories Inc., West Caldwel, NJ, USA) and Certified Reference Human Serum Material (Chengdu Shuyang Pharmaceutical Factory, Chengdu, China), obtaining similar (p > 0.05) mean Zn and Cu concentrations (0.789 ± 0.042 and 0.916 ± 0.016 mg/l, respectively) to the certified values (0.800 ± 0.060 and 0.900 ± 0.075 mg/l, respectively).
SPSS version 17.0 (IBM, Chicago, IL) was used for data analyses. The Kolmogorov-Smirnov test was applied to examine the normal distribution of variables and the Levene's test, to study the homogeneity of variances. Cu, Zn, and Cu/Zn ratio values were expressed as arithmetic means ± SD and were compared using the Student's t-test for parametric variables and the Kruskal-Wallis test for nonparametric variables. One-way analysis of variance was conducted to determine the influence on serum Zn and Cu concentrations and Cu/Zn ratios of CPI score, age, sex, death and disease complications. p < 0.05 was considered as statistically significant in all tests.
RESULTS
Mean serum Zn concentrations did not significantly differ between cirrhotic patients and controls (p = 0.196), while mean serum Cu concentrations were significantly higher in cirrhotic patients (p = 0.001) than in controls (1.263 ± 0.3250 vs 1.078 ± 0.097 ppm, respectively, p = 0.003), as were mean Cu/Zn ratios (Table I).
Table I. Mean serum Zn and Cu concentrations and Cu/Zn ratios in patients with cirrhosis versus healthy controls

Mean serum Zn concentration was significantly higher in the CPI-A group than in the CPI-B group (p = 0.025, Kruskall-Wallis test) (Table II), whereas these groups did not significantly differ in serum Cu concentration or Cu/Zn ratio (p > 0.05).
Table II. Mean serum Zn and Cu concentrations and Cu/Zn ratios in patients with cirrhosis by disease severity index measured by the Child-Pugh index (CPI) score

*The same superscript for values in the same column indicates a significant difference (p < 0.05).
The mean serum Cu concentration was significantly higher in the oldest age group (> 50 y; 1.360 ± 0.320 mg/l) than in the youngest (< 30 y; 1.130 ± 0.220 mg/l; p = 0.043). No significant differences in serum Zn concentration or Cu/Zn ratio were found among the three age groups of cirrhotic patients (p > 0.05) (Table III).
Table III. Mean serum Zn and Cu concentrations and Cu/Zn ratios in patients with cirrhosis as a function of death during the sampling period

*,†The same superscript for values in the same column indicates a significant difference (p < 0.05).
No significant difference was found between female and male cirrhotic patients in mean serum Zn or Cu concentrations or Cu/Zn ratios (p > 0.05; Student's-t test), although there was a non-significant tendency towards a difference between the sexes in mean Cu concentration and Cu/Zn ratio (p = 0.074 and p = 0.070, respectively; Student's t-test).
The mean serum Zn concentration was significantly lower (p = 0.02) and Cu/Zn ratio was significantly higher (p = 0.03) in non-surviving patients than in survivors during the one-year sampling period, whereas they did not significantly differ (p > 0.05) in mean serum Cu concentration (Table III).
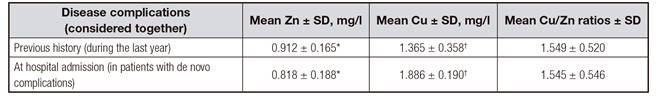
No differences were observed among the groups of complications recorded in patients (p = 0.067) (Table IV). When the two-tailed statistical test was applied, mean serum Zn values were significantly higher in patients with a previous history of ascites than in those with a history or presence at hospital admission of bacteremia-sepsis (p = 0.005 and p = 0.008, respectively) and in those with ascites at admission (p = 0.01). Higher mean Zn and Cu concentrations were observed in patients with a history of disease complications (considered together) than in those with their presence at hospital admission (p = 0.023 and p = 0.022, respectively) (Table V).
Table IV. Mean serum Zn and Cu concentrations and Cu/Zn ratios in patients with cirrhosis as a function of disease complications

*In the past (in patient with complications previously diagnosed during the routine checking by the hospital physician). +At hospital admission (in patients with de novo complications who would need hospital admission). ‡,§,ǁThe same superscript for values in the same column/row indicates a significant difference (p < 0.05).
DISCUSSION
In the present study, serum Cu concentrations and Cu/Zn ratios were higher in cirrhotic patients, while serum Zn concentrations were lower with greater severity of the disease. The mean serum Zn concentration was not significantly different between cirrhotic patients and controls (Table I), in disagreement with previous reports of significantly reduced serum Zn concentrations in patients with cirrhosis 3,32,33 and alcohol-related or other liver diseases 3,32. These findings of Zn deficiency may be explained by the frequent administration of diuretics to cirrhotic patients and their poor intestinal absorption of Zn 4,13. Other proposed explanations include the loss of Zn bound to albumin in the blood in liver disease or damage to the intestinal mucosa 11). Low serum Zn concentrations have also been related to protein reluctance or losses due to diarrhea and/or increased urinary excretion 3. Nangliya et al. 4 observed a significant decrease in serum Zn concentrations with more severe liver disease. Accordingly, our discrepant results may be attributable to the early stage of cirrhosis in the majority of our patients.
The mean serum Cu concentration was significantly higher in the patients than in healthy controls, in agreement with previous reports in patients with cirrhosis 3,4,6,32, chronic hepatitis C 34, and chronic hepatocellular carcinoma 2,23, among other liver diseases. Elevated Cu values have also been associated with nutritional abnormalities, oxidative stress, inflammation and immune dysfunction 21. It has been reported that nutritional disorders can impair biliary Cu excretion and thereby increase serum and hepatic concentrations of free Cu, which may produce injury from the resulting rise in oxidative stress 21. Excess Cu in the human organism has also been implicated in cell damage and the development of hepatocellular carcinoma 2.
The mean Cu/Zn ratio was significantly higher in cirrhotic patients than in controls, consistent with previous reports in patients with cirrhosis 2,21 or a digestive system, breast, lung, or hematological cancer, besides liver malignancies 23. The Cu/Znratio has been proposed as a useful biomarker of cirrhosis complications and liver disorders, among other diseases 22,25.
A significant reduction in mean serum Zn concentration was found with greater cirrhosis severity as measured with the CPI (p < 0.05), in agreement with previous reports 35. It has been previously reported that Zn deficiency is highly prevalent in cirrhotic patients with CPI-B or -C and is correlated with the severity of their disease 4. Zn deficiency may also play a critical role in the development of heart failure, and dietary supplementation and/or medical prescription of Zn for cirrhotic patients should not be ruled out 36. The lack of association between mean serum Cu concentrations and severity index is consistent with previously published results and may be attributable to the role of Cu in redox process 3,4). Our finding that Cu/Zn ratios were not related to cirrhosis severity is in disagreement with the report by Elzeiny et al. 37, possibly because only one patient in our series had the highest severity level. An elevated Cu/Zn ratio has also been associated with higher cardiovascular risk 26.
The age of the patients of this sample did not influence serum Zn concentrations or Cu/Zn ratios, as was previously observed by some authors 14), while others found significantly higher serum Zn concentrations in patients over versus under 60 years old 38,39. Another study in elderly people 39 reported Zn deficiency as well as cell-mediated immune dysfunction. In our patients, serum Cu concentrations were higher in patients over 50 than in those under 30 years of age. It has been reported that Cu overload may worsen cirrhotic disease and may impair other extrahepatic tissues, increasing the mortality risk in older patients 3,38.
Serum mean Zn concentrations did not differ between the male and female patients, as previously observed 3,38, but there was a tendency to slightly higher serum Cu and Cu/Zn values in the females. Grungreiff et al. 34 found higher serum Cu concentrations in female versus male chronic hepatitis C patients. Some authors have proposed that cirrhosis and the male sex may be involved in the progression of hepatocellular carcinoma 1.
Mean serum Zn concentrations were lower and Cu/Zn ratios were higher in patients who died from their disease during the study period. Zn deficiency could exacerbate liver damage by impairing antioxidant defense mechanisms 12, increasing the mortality risk, but more analysis must be done. Lin et al. 2 also reported elevated mean Cu/Zn ratios in patients with hepatic cirrhosis as well as in those with hepatoma, associating their increase with a greater risk of death from cardiovascular disease 24.
Serum Zn concentrations were higher in patients with than without ascites, a common symptom in patients with decompensated cirrhosis 1. Ascites have been found to produce muscle loss and a catabolic state in cirrhotic patients, leading to Zn losses 16. The administration of branched-chain amino was reported to reduce ascites in patients with liver cirrhosis 40.
In our opinion the main weakness of this study is the number of studied cirrhotic patients; in fact, for the CPI C group only one patient was considered and therefore, corresponding data were not included. Although serum Cu levels and Cu/Zn ratios were not influenced by the severity index of the liver disease, future studies should be performed with a higher and more balanced number of cirrhotic patients for the CPI A, B and C groups to elucidate definitively the hypothesis of this study. Inversely, its main strength is that, with the results obtained, we could expect that the Cu/Znratios could be useful as a predictive biomarker for cirrhosis disease status together with other complementary analysis.
Further research is warranted to determine whether the imbalance in antioxidant minerals observed in cirrhotic patients (increased serum Cu concentrations and Cu/Zn ratios and reduced serum Se concentrations [27]) may be directly related to the development of cardiovascular and renal disorders among others, as well as analysis related with oxidative stress or inflammation.
CONCLUSION
Serum Cu concentrations and Cu/Zn ratios are increased in cirrhosis. Serum Zn concentrations are significantly higher in the CPI-A group than in the CPI-B group. Serum Zn concentrations were lower and Cu/Zn ratios were higher in cirrhotic patients who died from the disease during the sampling period. These trace elements are essential during the progression of the disease so it is important control them. In addition, to the best of our knowledge, with the results obtained we could expect that the Cu/Zn ratios could be useful as a predictive biomarker for cirrhosis disease status together with other complementary analysis.