INTRODUCTION
Several adaptive mechanisms occur during pregnancy. Changes in the energetic metabolism are related to estrogen stimulation and insulin resistance. Physiologically, a 2-3 fold increase in plasma triglycerides (TG) is observed in the second and third trimester of pregnancy, accompanied by lower increases in total cholesterol (TC), high density lipoprotein (HDL) and low density lipoprotein (LDL). After delivery, TC, HDL and TG decrease while LDL tends to remain constant 1,2,3.
During the first two-thirds of gestation, hyperphagia, increased lipid synthesis and fat accumulation are characteristic 4,5, followed by a decrease in fat storage in the last third of gestation, related to enhanced lipolytic activity and decreased lipoprotein lipase activity in the adipose tissue 6.
Maternal serum TC and LDL are transported across the placenta, and this exchange provides cholesterol to the fetus, especially during early pregnancy 7,8. Cholesterol is required for cell proliferation and development of the fetus. Maternal hypertriglyceridemia also serves as an energy depot for maternal dietary fatty acids 9. TG do not cross the placental barrier, but the presence of lipoprotein receptors in the placenta, lipoprotein lipase, phospholipoproteins A2 and intracellular lipase activities allow the release of polyunsaturated fatty acids (transported as TG in maternal plasma lipoproteins) to the fetus 10,11. In addition, it has been suggested that serum TG values measured during the first third of gestation have predictive value for glucose tolerance during pregnancy 2. During pregnancy, an increased production of oxidisable particles and inoxidative damage are observed; these effects may be related to long-term cardiovascular risk, especially in those patients with other risk factors for cardiovascular disease 12. An association between the atherogenic lipid profile of gestation and the endothelial cell dysfunction during preeclampsia has been also described 13. Some other effects related to the offspring have been also published. Maternal cholesterol seems to affect fetal sterol metabolism and the metabolic functions of extra embryonic fetal tissues 14,15; for this reason, some studies have proposed clinical relations between maternal TG and increased birth weight (BW), especially in those pregnancies complicated with gestational diabetes (GDM) 16. However, the influence of maternal lipid metabolism on fetal growth and complications of pregnancy is still inconclusive. Although changes in lipid profile are expected, its regular evaluation during pregnancy has not been established yet, probably related to the lack of consensus regarding reference values per trimester and the diagnostic criteria for dyslipidemia (DLP).
Thus, considering the importance of maternal complications during/after pregnancy, the fetal development and BW, the aim of this study was to evaluate the presence of dyslipidemia in a high risk Spanish population with GDM using two different classification methods, and to analyze some putative associations between lipid profile, delivery, offspring characteristics and maternal metabolic syndrome parameters determined three and twelve months after delivery.
MATERIALS AND METHODS
PATIENTS
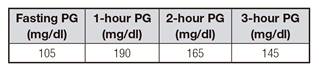
This study was conducted in accordance with the Declaration of Helsinki and with national and international guidelines. Two hundred and fifty patients with GDM who underwent endocrinological evaluation from 2013 to 2015 were included. Clinical records were used to collect full medical history; gestational age (GA) was obtained from estimates of gestational age during the first ultrasound (USG) when available or by the date of the last menstrual period; prematurity was defined as gestational age at birth between 24 and 36 weeks of gestation. A screening test for GDM with 50 g of glucose was performed in the first or second quarter of pregnancy, according to the clinical risk of patients following the current clinical guidelines 17. The diagnosis of GDM was performed according to a three-hour curve after the oral administration of 100 g of glucose (Supp. Table I) or a screening test with a plasma glucose > 200 mg/dl 18. In addition, blood samples including HbA1c, total cholesterol, LDL, HDL, triglycerides, TSH and kidney function were obtained during pregnancy and three months after delivery; the last one included a 75 g oral glucose tolerance test (OGTT). Fifty patients of the initial sample were also followed with the same biochemical analysis one year after delivery.
BIOCHEMICAL ANALYSIS
Blood samples were collected after 12-hour fasting. In pregnant women with more than one lipidogram collected during pregnancy, the first examination was considered for analysis. Glucose was determined using the glucose oxidase method, and kidney and lipid profile, using mass spectrophotometry; HDL and LDL were calculated.
DYSLIPIDEMIA DURING PREGNANCY
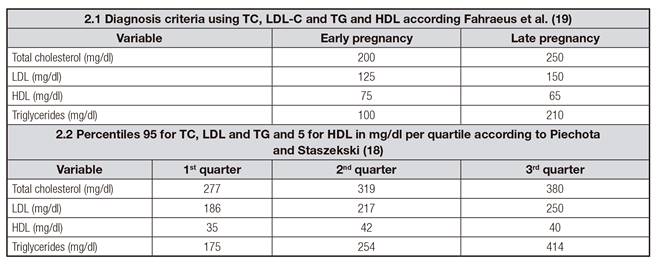
The prevalence of dyslipidemia was evaluated considering two definitions: a) classical dyslipidemia according to Fahraeus et al., where TC, LDL, HDL and TG concentrations are classified in early or late pregnancy 19 (Table 2.1); and b) percentile criteria according to Piechota and Staszekski (when there is elevation of TC, LDL and TG concentrations above the 95th percentile and HDL levels below the 5th percentile for gestational age) 20 (Supp. Table 2.2).
STATISTICAL ANALYSIS
Normality distribution of the data was determined using the Kolmogorov-Smirnov test. Continuous variables were presented as mean ± standard deviation. Categorical variables were reported in absolute and percentage values. Subjects were categorized as having dyslipidemia for each of the two criteria. Statistical differences between continuous variables with normal and non-parametric distribution were obtained using the unpaired Student's t-test and the Mann-Whitney U test, respectively. The Chi-squared and Spearman's correlation tests were used to compare categorical data. ROC curves were also performed; the area under the curve and the 95% confidence interval were also calculated. Statistical analyses were performed using SPSS statistical software v.20 and GraphPad Prism v.6. Data in graphs are expressed as mean ± SEM. p-values < 0.05 were considered as statistically significant.
RESULTS
PATIENT POPULATION AND CLINICAL CORRELATIONS
A total of 250 pregnant women with GDM were included. Demographic and clinical features of the general evaluated populations and their characteristics according to the presence of dyslipidemia are summarized in table I. The presence of dyslipidemia ranged from 27% to 86% using the Fahraeus et al. or the Piechota and Staszewski criteria respectively (Fig. 1). In both classifications, lower plasma HDL levels were the most current lipid disorder (9.9% and 61.9%, respectively) followed by hypertriglyceridemia (9.6% and 36.5%, respectively) (Fig. 1). Demographic and clinical characteristics were similar between patients with and without dyslipidemia according to both classification criteria. Insulin requirements tended to be increased in patients with dyslipidemia according to both classifications. Basal insulin requirements were statistically increased in dyslipemic patients according to the 3-quartile method (Piechota and Staszekski; p = 0.015). Prematurity and birth weight < 2,500 g or > 3,500 g were not correlated with the presence of dyslipidemia according to the classifications used (Table I).

Figure 1. Incidence of dyslipidemia during pregnancy according classical criteria (Fafreus et al., 1985) and 3-quartile classification (Piechota et al. 1992). TC: total cholesterol; LDL: low density lipoprotein cholesterol; HDL: high density lipoprotein cholesterol; TG: triglycerides; DLP: dyslipidemia.
Table I. Clinical and demographic characteristics of the evaluated population categorized according to the presence of any lipid alteration according to the classical criteria and the percentiles criteria
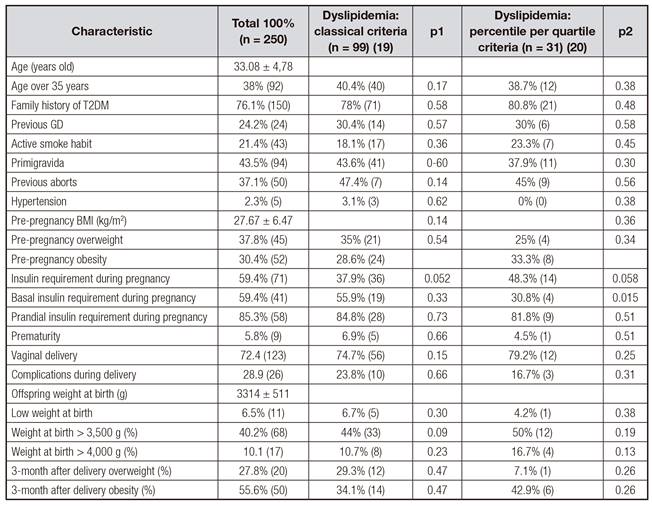
T2DM: type 2 diabetes mellitus; BMI: body mass index. p1 refers to relation with the presence of dyslipidemia according the classical definition; p2 refers to relation with the presence of dyslipidemia according the 3-quartile definition.
Patients who required insulin for controlling their blood glucose exhibited significantly decreased plasma HDL levels compared to those who did not require insulin (59 ± 5 vs 67 ± 3 mg/dl), and they also exhibited decreased plasma LDL levels (133 ± 7 vs 137 ± 11 mg/dl). Those patients requiring insulin had also lower plasma TC values (234 ± 12 vs 251 ± 9 mg/dl) (Fig. 2). Interestingly, elevated TC and LDL levels during pregnancy were related to vaginal delivery and normal OGTT 3-month after delivery (p < 0.05) (Fig. 3).
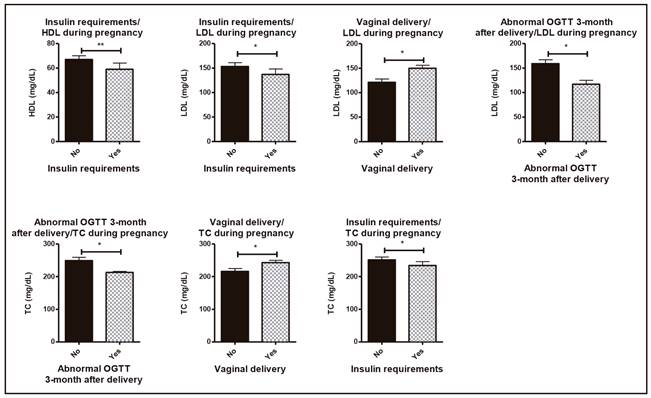
Figure 2. Clinical relations concerning pregnancy, metabolic syndrome characteristics and lipid profile during pregnancy. Asterisks (*p < 0.05; **p < 0.01).
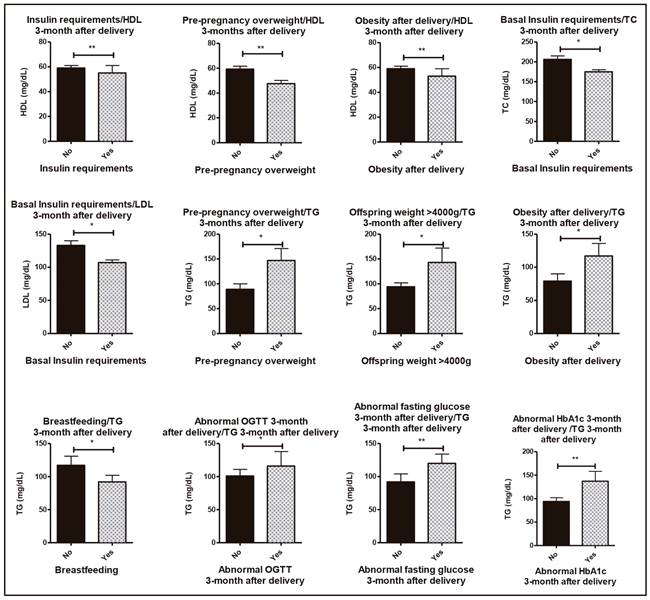
Figure 3. Clinical relations concerning metabolic syndrome characteristics and lipid profile 3-months after delivery. *p < 0.05; **p < 0.01).
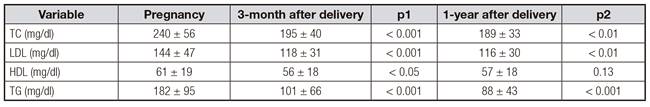
The lipid profile three months and one year after delivery is shown in table II. Concerning the lipid profile three months after delivery, importantly, decreased HDL levels were correlated to pre-pregnancy overweight, insulin requirement and obesity after delivery (p < 0.01). LDL and TC plasma values 3-month after delivery were lower in those patients that required basal insulin (175 ± 5 mg/dl and 107 ± 4 mg/dl, respectively). Women with pre-pregnancy overweight and newborns affected with macrosomia and obesity after delivery had increased TG values 3-month after delivery. On the other side, the presence of abnormal fasting glucose (plasma glucose > 100 mg/dl), abnormal OGTT (plasma glucose > 140 mg/dl) and abnormal HbA1c (> 5.7%) 3-months after delivery were correlated to increased TG levels in the same analysis (p < 0.05). Interestingly, those patients who breastfed their newborns exhibited decreased TG levels 3-month after delivery compared to those who used only artificial feeding (92 ± 10 vs 117 ± 14 mg/dl) (Fig. 3).
Furthermore, when patients were evaluated one year after delivery, those patients with pre-pregnancy overweight still had higher TG and lower HDL values. Decreased HDL values were also related to offspring weight > 4,000 g, abnormal fasting glucose and OGTT three months after delivery. TG > 100 mg/dl were observed in obese mothers and in those with abnormal fasting glucose 3-months after delivery. Breastfeeding was also related to decreased TG serum levels one year after delivery (Fig. 4). During pregnancy, the lipid-related parameter that better predicted the risk of offspring macrosomia was TG (AUC 0.655) (Fig. 5). This parameter was compared not only to other lipid profile serum parameters but also with other parameters related to metabolic syndrome including fasting glucose, OGTT, HbA1c and weight gain (data not shown).

Figure 4. Clinical relations concerning metabolic syndrome characteristics and lipid profile 1-year after delivery. *p < 0.05; **p < 0.01).
DISCUSSION
During pregnancy, although a physiological increase in TC, HDL, LDL and TG is expected, it is difficult to determine the cut-off level for diagnosing dyslipidemia (DLP) in pregnant women. It is important to early diagnose and identify those patients requiring intensive non-pharmaceutical intervention during and after pregnancy. This study shows the lipid profile behavior in Caucasian patients with gestational diabetes, and describes the presence of DLP according to two different classification methods, evaluating their utility to identify risk factors among patients with gestational diabetes.
The presence of dyslipidemia was significantly different according to the criteria used. However, the lack of association between the presence of DLP using these methods and the maternal/fetal outcomes raises questions about the clinical significance of measuring lipid profile during pregnancy. In the absence of evidence of cut-off points that early identify the risk population, probably the percentiles definition seems to better adjust to the lipid distribution during pregnancy. TG is apparently the best lipid parameter for identifying metabolic risk in pregnant women with GDM, while the evaluation of lipid profile after delivery provides useful information about the future risk of metabolic syndrome and cardiovascular complications. There is debate over the lipid patterns in gestational diabetes and whether they are potential markers on preexisting insulin resistance 21. In our study group, hypertriglyceridemia was one of the most prevalent modifications, as it has been described in pregnant women without GDM 16,22. One study in Spain reported an increased lipid profile in patients with GDM compared to pregnant women without GDM, which seemed to precede the diagnosis of GDM 23. Comparing our results to those of that study, in our group, similar TC values but increased serum TG are observed, suggesting an increased proinflammatory condition in our study population.
When pregnancy complications are considered, epidemiologic data suggest that women with proinflammatory phenotype tend to deliver preterm infants 24, and have a consequent increased risk for cardiovascular diseases 25. Some other studies have proposed that maternal hyperlipidemia could increase the oxidative stress in the fetus, resulting in vessel wall damage and disruption of normal placentation 26. In our study, no relation between lipid profile and birth date was observed.
The precise biological mechanism by which maternal cholesterol affects BW is still unknown. It has been suggested that altered sterol hormone metabolism and impaired cell cycle/signaling of growth factors including insulin could affect placental transport of nutrients influencing fetal growth 14,27. Some studies have described a relation between variations in maternal TG/weight and BW 28. In this sense, it has been suggested that not only BMI before pregnancy is related to BW 16, but also maternal adiposity and weight gain during pregnancy 28,29,30.
A meta-analysis found that women with gestational diabetes have elevated triglyceride concentrations compared to women without gestational diabetes 21, and these values, even the lipid profile, could be also influenced by lifestyle factors during pregnancy 31. In our group, triglycerides represented the best parameter to predict the presence of macrosomia, compared not only to lipid profile but also to the other metabolic syndrome parameters. At the same time, these mothers had significantly higher TG values three months after delivery.
There is limited knowledge on the relationship between maternal HDL and fetal development. HDL does not cross the placenta but it seems to influence cholesterol metabolism in the placenta, which could affect the size of the fetus 14. Related to this, HDL has been negatively associated to BW in some studies 30,32, including its rate of change among pregnancy 30. On the contrary, some others have reported no relations between HDL change during pregnancy and the BW adjusted by BMI 33. In our group, mothers with neonates > 4,000 g had decreased HDL levels one year after delivery, but not during pregnancy. Interestingly, the most prevalent lipid modification during pregnancy in our group was a decreased HDL value.
In our study, no relation between lipid profile and preeclampsia was found, as previously reported 16, but data concerning this cardiovascular risk factor is contradictory. Other publications, in contrast, suggest an association between hyperlipidemia, especially hypertriglyceridemia and preeclampsia 34. Concerning other complications, no relation between lipid profile and malformations was observed in our group. It has been reported that pregnancies complicated by both GDM and obesity have been associated with an increased rate of congenital malformations 23. A relation between congenital malformations and the oral administration of lipid peroxides generated from heated culinary oils has been described in animal models 35.
Pre-pregnancy BMI has been reported as the main factor related to changes in TC and LDL during pregnancy 36. In our cohort, patients with pre-pregnancy overweight had worse lipid profile (higher TG, lower HDL) even one-year after delivery, suggesting that the lipid profile during pregnancy obeys not only physiological changes but also the metabolic condition of the patient. Interestingly, there are contradictory publications about the effects of breastfeeding and lipid profile in young adults. It has been published that breastfed babies have better HDL/LDL ratio at six months compared to mix-fed babies 37. In our cohort, the lipid profile was not evaluated in the offspring, but breastfeeding was associated with healthier lipid profile in those mothers who breastfeed their children during the first three months of life, suggesting that breastfeeding may have benefits not only to the neonates but also to the mothers.
The association between impaired glucose tests 3-month after pregnancy and the HDL and TG levels one year after delivery confirms the close relation between glucose and lipid profile, and the importance of their parallel study in order to early identify and treat high-risk patients. This study adds to the current knowledge of associations between maternal blood lipid concentrations during pregnancy and outcomes for the baby.
This study had many strengths, including a prospective cohort evaluated 3-month and 1-year after delivery; anthropometric measurements were collected by trained healthcare professionals, and blood samples were analyzed according to a systematized protocol in our hospital. This task presents, however, some limitations. It is a unicentric study with pregnant women with GDM, the lipid profile is not compared to that of normal pregnant women, and the number of patients evaluated one-year after delivery was low. The results of the present study therefore do not allow national or international generalization and the absence of association with clinical outcomes. It would be interesting to follow up the metabolic behavior of the offspring, as well as the presence of cardiovascular complications in women with dyslipidemia during pregnancy. At the same time, future research should examine whether lipid abnormalities in the first trimester are useful to identify patients who will develop GDM.