INTRODUCTION
While hypertensive have lower endothelial function for nitric oxide (NO) synthesis 1, and greater sympathetic activity at rest 2, L-arginine supplementation for four to 24 weeks promotes blood pressure (BP) reduction 3,4,5,6. These authors argue the low endothelial capacity for NO production in patients before supplementation, which was explained by the low bioavailability of the L-arginine substrate, so that supplementation stimulated an improvement in endothelial activity 6,7 and improved blood flow 8.
A single session of aerobic exercise can promote a significant reduction of BP, a phenomenon called post-exercise hypotension (PEH) 9. Exercise increases blood flow in order to promote a shear stress phenomenon which induces NO production 10,11. But no studies evaluated whether an increased production of NO would also accompany BP reduction with L-arginine supplementation.
Even though exercise is able to increase NO synthesis and, consequently, results in a reduction of post-exercise BP, low endothelial capacity may limit this response. Although previous data show that chronic L-arginine supplementation protocols reduce BP and increase NO production at rest, it is not known whether ingestion of a single dose of this aminoacid prior to an exercise session would also increase NO production in response to shear stress exercise-induced. The practical implication of this is that a possible increase in NO production could potentiate PEH.
Therefore, the aim of this study is to verify if a dose of L-arginine improves post-exercise hypotension, increases NO production and reduces oxidative stress o in middle-aged hypertensive patients.
MATERIALS AND METHODS
SUBJECTS OF STUDY AND ETHICAL ISSUES
The study was conducted with 20 diagnosed hypertensive patients (51.47 ± 1.24 years), five men, previously sedentary, who did not use nitrate drugs, betablockers and calcium channel blockers, no history of supplementation with L-arginine, non-diabetics, and non-menopausal women. Subjects who changed the medication and missed at least one of the experimental protocols were excluded. The project was previously approved by the ethics committee in research with human beings of the University Hospital Lauro Wanderley, under protocol 625/10. All subjects were asked to sign the free and informed consent form according to Resolution 466/12 of the National Health Council.
STUDY DESIGN
This was a randomized, double-blind, placebo-controlled clinical trial. Three procedures were performed with 48-hour intervals: two sessions of aerobic exercise with previous ingestion of L-arginine (EX-LARG) or placebo (EX-PLA) and one with L-arginine intake without exercise (L-ARG) (www.randomizer.org). BP was recorded at rest and every ten minutes during a recovery period of 60 minutes after exercise or in an equivalent period in the non-exercise procedure. Blood samples were taken at rest and immediately after exercise or at equivalent times in the protocol without exercise for further analysis of the serum concentration of malondialdehyde (MDA) and nitrite.
ADAPTATION OF THE EXERCISE PROTOCOL
Volunteers performed three to five adaptation sessions at intervals of at least 24 hours until they were able to walk for 60 minutes at a minimum intensity of 60% of the maximum heart rate (MHR), which was estimated from the Bruce equation (1974) 12.
EXERCISE PROTOCOL
Twenty-four hours after the last adaptation session the volunteers were invited to start the treadmill exercise lasting 60 minutes. The volunteers were instructed to stay in the target training zone, prescribed by the researchers and characterized by intensity between 60% and 85% of the MHR, as proposed by Karvonen (1957) 13. The intensity was controlled by the heart rate (HR) measurement using a Polar RS800CX(r) cardiometer (Polar ElectroOy, Kempele, Finland) and subjective effort perception through the scale proposed by Borg (1982) 14, with a score of 6 to 20. These measures were performed every ten minutes during the physical exercise. The sessions were performed in the afternoon, between 1 pm and 6 pm.
SUPPLEMENTATION PROTOCOL
L-arginine supplementation and the placebo were produced in a handling laboratory and previously passed a certification examination. Seven grams of lemon-flavor L-arginine or equally flavored placebo (Starch), both diluted in 100 ml of water, were administered. This amount of L-arginine was based on the study by Lima et al. (2012) 6. The ingestion took place 30 minutes before the start of the experimental sessions. The volunteers were instructed not to ingest food sources of L-arginine for a period of 24 hours before each session. Accordingly, they received a list of foods rich in L-arginine that should be avoided in this period.
MEASURES OF BLOOD PRESSURE
Blood pressure measures were recorded after ten minutes at rest in the sitting position, immediately at the end of the exercise and every ten minutes during the recovery period of the exercise (60 minutes) or in an equivalent period in the non-exercise procedure. BP measurements were performed by the auscultatory method and always by a single evaluator throughout the whole data collection process, following the VI Brazilian Guidelines for Hypertension of the Brazilian Society of Cardiology, Brazilian Society of Nephrology and Brazilian Society of Hypertension 15. The instrument used was a Welch Allyn(r) aneroid sphygmomanometer (Welch Allyn Inc., New York, USA), previously calibrated against a mercury column.
BIOCHEMICAL ANALYZES
Blood samples were taken at rest (before L-arginine or placebo ingestion) and immediately after exercise or at equivalent times in the non-exercise protocol for further analysis of the serum concentration of MDA and nitrite. Six ml of blood were collected from the antecubital vein in heparinized tubes and centrifuged at 3,000 rpm for 20 minutes. The plasma was separated and refrigerated until analysis.
Plasma concentration of nitrite was measured using the Griess reagent method. The reagent was prepared using equal parts of 5% phosphoric acid, 0.1% N-1-naphthylenediamine (NEED), 1% sulfanylamide in 5% phosphoric acid and distilled water. For the assay, 100 μl of the 10% homogenate supernatant, made with potassium phosphate buffer, in 100 μl of the Griess reagent were added. To the blank, 100 μl of the reagent were added in 100 μl of buffer and to obtain the standard curve serial dilutions (100, 50, 25 12.5, 6.25, 3.12, 1.56 μM) of nitrite were made. The entire assay was done on a 96-well plate and the reading was made in the absorbance range of 560 nm.
The evaluation of the oxidizing activity was quantified by means of the reaction of the thiobarbituric acid or MDA with the products of decomposition of the hydroperoxides. For this, 250 μl of the sample were added to KCl and incubated in a 37 °C water bath for 60 minutes. Thereafter, the mixture was precipitated with 35% AA perchloric acid and centrifuged at 14,000 rpm for ten minutes at 4 °C. The supernatant was transferred to new ependorfs and 400 μl of 0.6% thiobarbituric acid were added and incubated at 95-100 °C for 30 minutes. After cooling, the material was read in a spectrophotometer at a wavelength of 532 nm.
STATISTICAL ANALYSIS
Data were initially tested for normality and homogeneity by the Shapiro-Wilk and Levene tests. One-way ANOVA tests were used to compare the baseline conditions on the four days of procedures and two-way ANOVA was used to evaluate possible differences in pressure responses and autonomic variability between procedures, always adopting a 95% confidence level (p < 0.05). Instat 3.0 software (GraphPAd Instat, San Diego, CA, USA) was used. The program GPower 3.1.7 was used to calculate the effect size 16.
RESULTS
Table 1 presents the conditions of the volunteers. The volunteers were obese (32.64 ± 5.20 kg/m²) and had no diagnosis of diabetes or other cardiovascular diseases besides hypertension. All were treated with antihypertensive medication and on the days of the experimental procedures, they presented pressure values compatible with controlled blood pressure levels. The values of BP, HR, nitrite and MDA were statistically similar at these moments prior to the study procedures.
Table I. Conditions of the study variables at the moments preceding the experimental procedures

RHR: resting heart rate; RSBP: resting systolic blood pressure; RDBP: resting diastolic blood pressure; MDA: malondialdehyde. Data is average and standard error. There were no statistically signifcant differences between the rest data (p < 0.05).
In the exercise sessions, they reported between 9.60 ± 2.47 and 13.11 ± 3.38 points on the effort perception scale, as shown in Figure 1. Despite having performed the exercises within the range suitable for perceiving effort, the intensity measured by the HR was only between 28.28% and 50.60% of the MHR with a mean of 41.10%. In any case, the two exercise sessions were performed with intensities quite similar to each other.

Figure 1. Subjective effort perception and heart rate behavior during the exercise sessions of the experimental procedure. Data is average and standard error. *Statistical difference of the L-ARG procedure for EX-LARG and EX-PLA procedures (p < 0.0001, effect size = 2.45).
The Figure 2A presents the difference between the mean of the six post-exercise BP measures and the baseline values for the three procedures performed. In the two procedures in which the volunteers exercised, a statistically significant mean BP reduction of -6.58 ± 0.95 mmHg and -8.38 ± 1.29 mmHg, respectively, occurred for EX-LARG and EX-PLA in relation to the pre-exercise value. Meanwhile, the mean pressure value of the six measures of the moments corresponding to the post-exercise value of the L-ARG procedure (+4.30 ± 1.12 mmHg) was significantly higher in relation to the exercise procedures. These data demonstrate the occurrence of PEH after exercise, with effect size calculated at 3.0 and 2.6 for EX-LARG and EX-PLA, respectively. However, when EX-LARG and EX-PLA are compared, there is no statistical difference between these procedures (p = 0.28). In Figure 2A, details of BP values can be visualized by the variation between the values every ten minutes post-exercise and the baseline moments. EX-LARG and EX-PLA were significantly lower in relation to the L-ARG procedure after 40 min post-exercise. However, no differences were noted between the two exercise procedures (EX-LARG and EX-PLA) at any of the post-exercise BP measurement moments.

Figure 2. Delta values of SBP and DBP between rest and post-exercise recovery period. Data is average and standard error. *Statistical difference of the L-ARG procedure for the EX-LARG and EX-PLA procedures. **Statistical difference of the EX-LARG procedure for EX-PLA and L-ARG procedures.
Regarding the response of the post-exercise diastolic component, we observed that the mean of the six post-exercise blood pressure measurements in relation to baseline EX-LARG, EX-PLA and L-ARG values was -1.85 ± 0.44 mmHg, +2.13 ± 0.62 mmHg and +3.81 ± 0.79 mmHg, respectively (Fig. 2B). Descriptively, it is noted that PEH occurred only in the EX-LARG procedure, which is statistically confirmed in both the EX-LARG comparison for EX-PLA and the EX-LARG for L-ARG with effect size of 0.82 for the hypotensive effect of EX-LARG. Figure 2B shows the diastolic behavior in detail for the variation between each post-exercise measure with the baseline values. By this analysis, the pressure reduction observed in the EX-LARG procedure was significantly lower than EX-PLA at 20 minutes post-exercise. However, in the other moments, a statistical trend was observed for the lowest values observed in EX-LARG (p < 0.10). In fact, some effect size was noted in all measures, ranging from 0.14 to 50 minutes up to 0.82 at 20 minutes post-exercise.
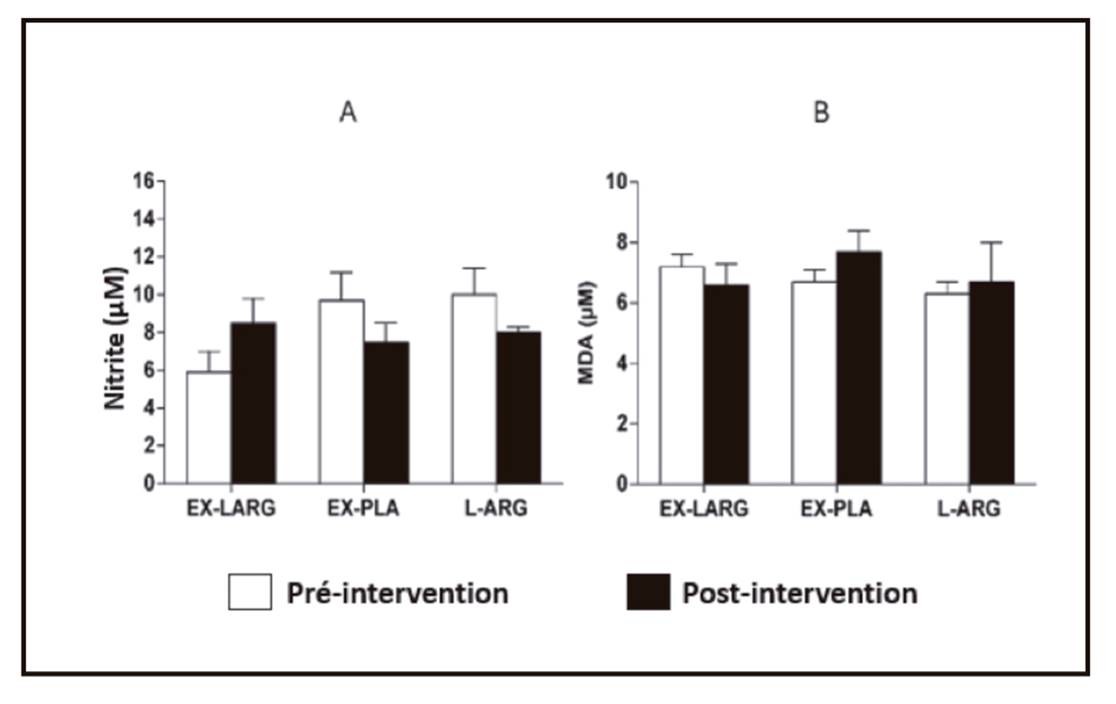
`Figure 3 shows the serum concentrations of nitrite and MDA in response to the experimental procedures. The EX-LARG and L-ARG procedures were not able to significantly alter nitrite and post-exercise MDA concentrations. However, it should be noted that while the EX-PLA procedure resulted in serum nitrite reduction (at a descriptive level and with effect size of 0.52), the exercise of the EX-LARG procedure resulted in an inverse behavior, with a descriptive increase of the nitrite concentration post-exercise and effect size of 1.0, which is almost 100% greater.
DISCUSSION
The data from this study show that ingestion of a dose of seven grams of L-arginine prior to an aerobic exercise session does not influence systolic BP and promotes a discrete but significant post-exercise diastolic hypotensive response compared to exercise performed without prior ingestion of L-arginine. L-arginine induces increases on post-exercise nitrite, while exercise alone results in decreased nitrite.
Reduction of resting BP after chronic L-arginine supplementation protocols has been pointed out in original studies 3,4 and is ratified in a meta-analysis by Dong et al. (2011) 5, where the authors used only studies that were randomized, double-blind and with control, with a total of 387 subjects who ingested L-arginine from four to 24 weeks and obtained significant reductions of 5.39 mmHg for systolic pressure and 2.66 mmHg for diastolic pressure. While in these studies the ingestion of L-arginine was chronic and BP data refer to the resting state, in the present study the effect of a single dose on post-exercise hypotension was evaluated. The unprecedented nature of this study makes it impossible to compare our results with previous data, but it supports the hypothesis that L-arginine could influence post-exercise pressure responses acutely.
Although the mean of the six post-exercise BP values (Fig. 2B) statistically indicated diastolic hypotensive response induced by previous L-arginine ingestion in comparison with the other procedures, statistical differences were only noticed at 20 minutes. However, effect size of at least 0.14 was found. The implication of this is that a more consistent post-exercise diastolic hypotension induced by prior L-arginine ingestion would be better demonstrated with a sample of 34 people. Another parallel aspect that should be noted in relation to the magnitude of the post-exercise hypotensive response is that the volunteers started the procedures with well controlled BP. This may be a result of concomitant drug treatment and has not been discontinued during the study. Therefore, studying hypertensive patients with higher BP values and impaired endothelial function may result in a different effect than that found in this study.
Regarding the possibility of intrinsic vasodilatation, it could be determined by the vasodilatory agents derived from the vessel itself, of which it is known that the endothelial production of NO is the most potent and that L-arginine is the precursor of NO 17. Although the data have not shown statistical significance, an effect size almost 100% higher in the EX-LARG procedure than in the EX-PLA was noted. This indicates that an increase in sample size may statistically confirm this acute but only descriptive improvement of nitrite induced vasodilation resulting from the administration of a single dose of L-arginine prior to an exercise session. Chronically, Lima et al. (2012) showed that nitrite concentrations after exercise with chronic supplementation (four weeks) of L-arginine increased significantly by almost twice, accompanying the reduction of rest BP, and these data corroborate a previous study 3.
The present study has some limitations, such as the fact that volunteers were expected to perform exercise with intensity between 60% and 85% of maximum heart rate, but they did so below this range for at least the first half of the session. The causal factor was that they reported subjective perception of effort compatible with the desired intensity and refused to increase the speed of the treadmill until they reached the predicted heart rate. From the perspective of the observation of the effect of L-arginine, this may not represent an intervening factor, but the magnitude of the PEH may have been influenced by this methodological phenomenon. Finally, it should be considered that the study was done with non-menopausal women, so that the menstrual period as well as the use of a contraceptive could influence the results of the study. However, while there is no body of evidence regarding this possibility, previous studies 18,19 indicate that menopausal and non-menopausal women had the same effect of a training program physicist. However, in these, the chronic effect of the training was verified, whereas in the present study, acute responses to an exercise session were evaluated. Thus, although these influencing aspects were not considered in the present study, they cannot be discarded.
The implications that can be drawn from the data of this study are practical and scientific. The practical implication represents the unprecedented finding that the administration of L-arginine could be useful to improve the hypotensive effect of hypertensive patients who adopt physical exercise as a therapeutic tool. The scientific implication is that the data from this study are unprecedented, but the magnitude of the effect found was discrete, and only for diastolic blood pressure. This represents the need for these data to be confirmed with other doses of L-arginine, more intense exercise protocols and hypertensive patients with higher resting blood pressure levels. Anyway, this study is the first to point out the possibility that a nutritional supplementation with L-arginine may enhance the antihypertensive potential of physical exercise.
CONCLUSION
Taken together, data from this study showed that L-Arginine intake promotes an improvement in the diastolic component of PEH, without altering the systolic component. The data from this study indicate that increased nitrite production may be an explanatory mechanism for the best diastolic hypotensive response.