INTRODUCTION
Telomeres are cellular structures composed of repetitions of DNA sequences (TTAGGG), located at the end of chromosomes; their function is to protect chromosomes from degradation during each cell cycle (1). Telomerase is the enzyme responsible for the maintenance of TL, and its function is to add TTAGGG repeats to the 3’ end of the sequence by retrotranscription (2). The largest telomerase activity is observed mainly in gametes, stem cells, and tumor cells (3). In somatic cells during cellular replication, TL shortens gradually, and this shortening gives rise to mutagenesis and chromosomal instability; as a consequence, there is tumorigenesis when excessive shortening occurs after reaching a certain threshold (4). TL may reflect the effects of behavioral, psychosocial and environmental factors on health status, and may predict morbidity and mortality (5). Short TL has been linked to age-related diseases and NCDs. In epidemiological studies telomere shortening has been associated with risk for various cancers, such as bladder (6), gastric (7), colorectal (8), and breast cancer (9,10).
Telomere shortening has been related to high levels of inflammation (11), oxidative stress (12-14), and metabolic factors such as abdominal fat, elevated blood glucose levels, and hypertension (15,16). A shorter TL has also been linked to conditions associated with lifestyle that are potentially modifiable factors, such as a decrease in fruit consumption (7) and physical inactivity (17,18). A longer TL has been linked with having a healthy diet, moderate alcohol consumption, maintaining a healthy body weight, abstaining from smoking, and participating in moderate or vigorous physical activity (19). Thus, findings from different studies show that lifestyle changes, including healthy dietary patterns and an increase in physical activity, may decrease telomere shortening. However, some studies have not found such associations, which leads to contradictory results regarding the effect of physical activity or diet on TL. The aim of this review is to examine the results of human studies that evaluated the role of lifestyle factors, such as dietary patterns, nutrients and physical exercise, on the promotion of TL changes. We also discuss the possible mechanisms of action that influence this process, with the perspective that TL could be a novel biomarker, measurable in blood samples, to indicate the risk of suffering age-related diseases or NCDs; it could be useful for promoting healthy lifestyles in the population. TL is highly variable among tissues and blood cell subpopulations due to their different proliferative history (20). Therefore, the studies that were included in this review measured TL from whole blood, buccal cells, and peripheral blood mononuclear cells.
The studies included in this review were identified by a literature search conducted in the PubMed Central database. The following keywords were used as search criteria: “telomere length and nutrients” OR “diet” OR “antioxidants” OR “micronutrients” OR “food” OR “vitamins” OR “exercise” OR “physical activity”. We conducted the last search in December 2018. The search included cross-sectional designs, case-control and cohort studies, and clinical trials. We considered studies involving male or female adults, healthy or with any condition such as cancer, diabetes, hypertension, or overweight and obesity. Among the scientific articles dealing with diet or physical activity included in this review, thirty describe the effect of diet on TL (Table I), fifteen evaluate the effect of physical activity (Table II), and five focus on the effects of both diet and physical activity on TL (Table III). The majority of studies are cross-sectional (twenty-nine), eight studies are cohort studies, ten are randomized controlled trials, one is a clinical trial, and two are case-control studies.
Table I. Effect of diet on telomere length

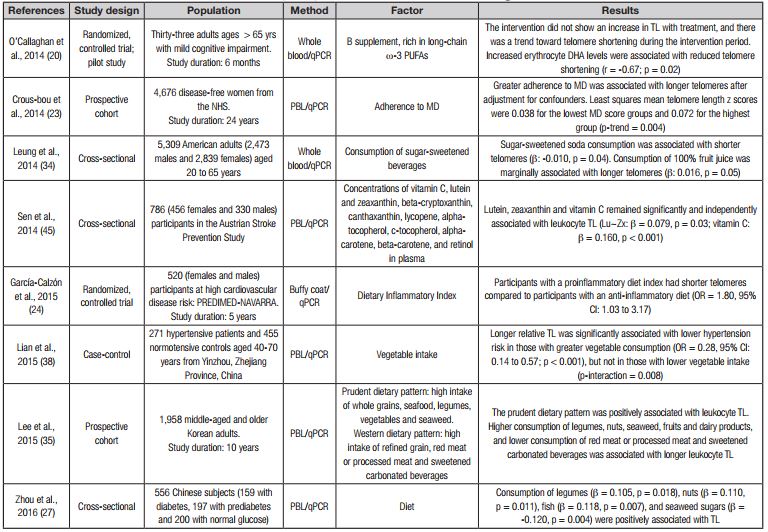

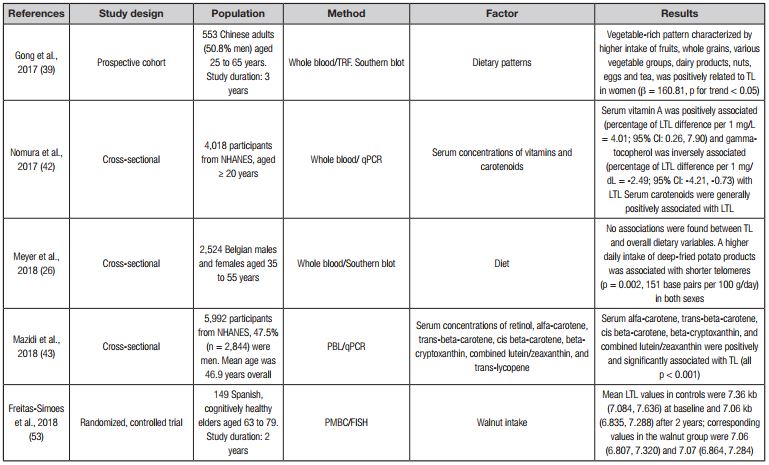
DII: dietary inflammation index; TL: telomere length; NHS: Nurses’ Health Study; F: Fisher's test; β: regression coefficient; OR: odds ratio; PREDIMED: Prevention with Mediterranean Diet; qPCR: quantitative polymerase chain reaction; TRF: telomere restriction fragment; T/S: the ratio of telomere PCR value to single-copy gene value derived from quantitative PCR; FISH: fluorescence in situ hybridization; PBL: peripheral blood leukocytes; PMBC: peripherial blood mononuclear cells; NHANES: National Health and Nutrition Examination Survey; BMI: body mass index; SFA: saturated fatty acids; PUFA: polyunsaturated fatty acids; EPA: eicosapentaenoic acid; DHA: docosa-hexaenoic acid.
Table II. Effect of physical activity on telomere length


TL: telomere length; NHS: Nurses' Health Study; β: regression coefficient; OR: odds ratio; CRP: C-reactive protein; qPCR: quantitative polymerase chain reaction; TRF: telomere restriction fragment; T/S: the ratio of telomere PCR value to single-copy gene value derived from quantitative PCR; PBL: peripheral blood leukocytes; PMBC: peripheral blood mononuclear cell; NHANES: National Health and Nutrition Examination Survey; ALPHA: Alberta Physical Activity and Breast Cancer Prevention; BMI: body mass index; MET: metabolic equivalent of task; HDL: high-density lipoprotein; CVD: cardiovascular disease.
Table III. Joint effect of diet and physical activity on telomere length

PBMC: peripheral blood mononuclear cells; TL: telomere length; PBL: peripheral blood leukocytes; qPCR: quantitative polymerase chain reaction; T/S: the ratio of telomere PCR value to single-copy gene value derived from quantitative PCR; LDL: low-density lipoprotein; HDL: High-density lipoprotein; IQR: interquartile range.
DIET AND DIETARY PATTERNS LINKED TO TELOMERE LENGTH
Several studies reported that adherence to dietary patterns or different dietary components, such as consumption of fiber, antioxidants, fatty acids and vitamins, as well as consumption of food groups such as fruits, vegetables, nuts, seeds, legumes, fish, sweetened beverages, and others, may be related to changes in telomere attrition (Table I). Dietary patterns describe the eating habits of a population; examples include MD, western diet, vegetarian diet, vegan diet, and others. Dietary patterns also reflect adherence to the formal dietary guidelines recommended for disease prevention (21). MD is a healthy diet that has been studied as protective against various chronic diseases (22), and is characterized by consumption of fruits and fresh vegetables, fish, cereals, vegetable fibers, nuts, and low saturated fat. Crous Bou et al. (23) conducted a study involving 4,676 healthy American women, and greater adherence to MD was significantly associated with longer TL. Boccardi et al. (15) reported that older adults without hypertension, myocardial infarction, vascular disease, dementia, stroke or heart failure, and with greater adherence to MD had higher telomerase enzyme activity and consequently longer TL as compared to those with low adherence to MD. In addition, participants also had low levels of some inflammation biomarkers such as C-reactive protein (CRP), interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α), as well as low levels of nitrotyrosine.
The pro- or anti-inflammatory potential of diets has been studied in relation to TL using the dietary inflammation index, which is a tool for assessing a diet’s inflammatory capacity; it is thus a population-based index representing a refined scoring algorithm based on an extensive review of the literature. Dietary variables are classified and scored according to their pro-inflammatory effect, anti-inflammatory effect or no effect on inflammatory biomarkers such as, IL-1b, IL-4, IL-6, IL-10, TNF- α, and C-reactive protein (24,25). García Calzon et al. (24) evaluated whether a diet associated with inflammation could modify telomere attrition rate by applying an intervention where MD was used for 5 years in 520 participants at high risk for cardiovascular disease. At baseline, the authors found that participants who had an anti-inflammatory diet had longer telomeres compared to those with pro-inflammatory diets. In addition, after 5 years of follow-up, participants with a pro-inflammatory diet had a 2-fold higher risk of accelerated telomere attrition when compared to participants with an anti-inflammatory diet.
Likewise, a cross-sectional study carried out by Shivappa et al. (25) in American adults evaluated the inflammatory potential of diet from a calculation of the dietary inflammation index obtained by the implementation of 24-hour recalls to participants. This study found that the highest dietary inflammation index scores were associated with telomere shortening. Another study reported no significant associations between TL and dietary inflammatory index or daily energy and fiber intake (26). The findings of these investigations suggested limited results of association studies between dietary patterns and TL. On the other hand, in a cross-sectional study in Chinese adults with diabetes, prediabetes, or normal glucose, Zhou et al. (27) evaluated the influence of diet on leukocyte TL, inflammation markers, and oxidative stress. The authors reported that consumption of legumes, nuts, fish and seaweed are factors associated with reduced telomere shortening, since they found a direct association between consumption of these foods and greater TL.
Contradictory findings have emerged on the consumption of meats and fats, and on their association with TL. In the study by Zhou et al. (27) consumption of meat, fat, and carbohydrates was not associated with telomere shortening but with an increase in inflammation as determined by TNF-α and IL-6 levels. Meanwhile, Fretts et al. (28) found that the consumption of processed meats was associated with a telomere shortening rate of 0.02 units per additional serving consumed per day. This finding is similar to that reported by Nettleton et al. (29), who found that for each additional serving/day of processed meat, the ratio of quantitative polymerase chain reaction telomere to single-copy gene (T/S ratio) was smaller by 0.07. Fretts et al. (28) describe a possible mechanism related to this finding, which is that processed meats have high concentrations of advanced glycation products, which are formed during the processing of meat and have been associated with telomere shortening by its ability to induce high levels of inflammation and oxidative stress. In addition, the authors refer to the importance of paying attention to the portion and frequency of consumption, not only to type of food. In this sense, Kasielski et al. (30) reported that consumption of red meats 1 to 2 times per week is associated with an increase in TL; this finding differs from those published by Fretts et al. (28) and Nettleton et al. (29), who reported that daily intake of meat is associated with telomere attrition.
In an intervention study to promote lifestyle changes in adults, the authors found that healthy changes (diet rich in whole grain foods, vegetables, fruits, and low-fat proteins, and moderate aerobic exercise, stress management, and increased social support) at 5 years of follow-up were associated with an increase in relative TL in the intervention group versus controls (31). Meanwhile, Hovatta et al. (32) obtained different results, since they reported that a lifestyle intervention during a 4.5-year follow-up period of weight loss, increased physical activity, and healthy diet did not have an effect on TL. Similarly, Bethancourt et al. (33) found no associations between blood TL and body mass index or dietary factors such as processed meats, fried/grilled meats and fish, nonfried fish, coconut oil, fruits, vegetables, bread products, and sugar-sweetened beverages. The consumption of the latter was associated with telomere shortening in a cross-sectional study (34). On the other hand, Lee et al. (35) evaluated the association between two dietary patterns and TL in the remote past. The first one was called a prudent dietary pattern, characterized by a high intake of whole grains, seafood, legumes, vegetables and seaweed. The second was the Western dietary pattern, characterized by a high intake of refined grain, red or processed meat and sweetened carbonated beverages. Consistent with previous findings, Lee et al. found that high adherence to a prudent dietary pattern was positively associated with TL. In addition, according to the analysis of particular food items, consumption of products such as legumes, nuts, seaweed, fruits and dairy products, and lower consumption of red meat or processed meat and sweetened carbonated beverages, were associated with longer TL (32,34).
Several studies report that dietary patterns with a high consumption of fruits and vegetables are related to an increase in TL (36-39). However, fruits and vegetables are taken as a whole group, and no particular type of fruit or vegetable is evaluated. Thus, it is difficult to attribute the aforementioned effect to a specific fruit or vegetable. A broader view is observed in the study conducted by Marcon et al. (37), who reported that a higher intake of vegetables was related to a higher mean TL, and the effect was specifically attributed to the intake of root vegetables, peppers and carrots. In an interesting study involving diet, TL and disease, Lian et al. (38) assessed the relationship between consumption of fruits and vegetables with TL in normotensive and hypertensive adults. In normotensive participants, increased intake of vegetables was associated with increased age-adjusted TL. In addition, the authors found that a longer TL was associated with a reduced risk of high blood pressure in participants with a higher consumption of vegetables. These data suggest that a high intake of vegetables in the diet promotes improvement in biomarkers such as TL, which can serve as a prognostic marker for cardiovascular disease.
MICRONUTRIENTS, ESSENTIAL FATTY ACIDS AND TELOMERE LENGTH
Epidemiological studies report a direct association between dietary micronutrients and TL (Table I), including the study by Marcon et al. (37), in which the analysis of the association between micronutrients and mean TL highlighted a significant role of antioxidant intake, especially beta-carotene, on telomere maintenance. Likewise, Yabuta et al. (40) reported that high intake of dietary beta-carotene and alpha-tocopherol protects buccal cells from telomeric shortening. In addition, carotenoids and other micronutrients, such as vitamin C, folate and potassium, have been positively associated with TL in Korean populations according to Lee et al. (41).
In another aspect of dietary information, there are studies that evaluated the relationship between serum or plasma concentrations of carotenoids or vitamins with TL (Table I). A study by Nomura et al. (42) reported that serum carotenoids are positively associated with leukocyte TL. Additionally, Mazidi et al. (43) reported that serum α-carotene, trans-β-carotene, cis-β-carotene, β-cryptoxanthin and combined lutein/zeaxanthin were positively and significantly associated with TL. Min and Min (44) only found a significant association of provitamin A carotenoids, such as alpha-carotene, beta-carotene (trans + cis), and beta-cryptoxanthin with TL; they found no association between nonvitamin A carotenoids (combined lutein/zeaxanthin and trans-lycopene) and TL. Another study provides evidence that higher plasma lutein, zeaxanthin, and vitamin C concentrations are associated with longer TL (45). Furthermore, plasma or serum 25-hydroxyvitamin (vitamin D) levels from diet (46,47) and supplements (48,49) were positively associated with leukocyte TL, while in another study plasma vitamin D concentrations from the diet were not associated with relative leukocyte TL (50).
Studies that consider omega-3 and omega-6 fatty acids cannot be ignored. Thus, in a study performed in adults with coronary heart disease, Farzaneh-Far et al. (51) reported that the consumption of marine omega-3 fatty acids after 5 years of follow-up increased blood omega-3 levels, an increment that was associated with a 32% decrease in the odds of telomere shortening. Indeed, two randomized controlled trials evaluated the impact of omega-3 fatty acid supplementation on telomere shortening. The first trial (52) included 138 participants and demonstrated that supplementation with omega-3 fatty acids has an impact on reducing telomere shortening; the authors suggest the importance of considering the ω-6:ω-3 PUFA ratios for future nutritional interventions. The second trial that evaluated supplementation with omega-3 fatty acids (20) did not show an increase in telomere length, probably due to a smaller sample size (33 participants) as compared to the study by Kiecolt-Glaser et al. (52). In another controlled randomized trial the intake of nuts for two years in older individuals tended to delay telomere attrition compared with individuals with a usual diet without nuts; the authors suggest that nuts are rich in omega-3 fatty acids and other antioxidants that might have an impact on the aging process (53).
There are few studies on the association between micronutrients and telomere length, and a majority of such studies have evaluated intakes of vitamin A, C, D, carotenoids, and omega-3 fatty acids. However, other micronutrients may be found in the diet, such as folate, potassium and zinc that could be related to cellular aging. The information compiled in this review highlights the importance of promoting further intervention studies that include supplementation with specific micronutrients and other diet components to determine their causal relationship with changes in TL.
PHYSICAL EXERCISE AND ITS LINKAGE TO TELOMERE LENGTH
As previously described, physical activity has positive effects on TL. In this way, some authors reported that only moderate to vigorous physical activity, which includes walking briskly, jogging, running, bicycling, swimming, tennis, and aerobics, can reduce telomere shortening (54-56). Nevertheless, Ludlow et al. (57) reported that moderate physical activity has a positive relationship on TL, and this effect is lost at higher physical activity levels. Loprinzi and Sng (58), working with data from the National Health and Nutrition Examination Survey (NHANES), reported that running was the only type of physical activity that was positively related to leukocyte TL. A recent study from NHANES performed by Tucker (59) reported that participants with a sedentary lifestyle had 1.95 times the likelihood of having short telomeres compared to those with high physical activity; the results obtained for sedentary patients did not differ for low or moderate levels of activity. Fretts et al. (60), in a cross-sectional study, found that ambulatory physical activity measured by the number of steps taken per day, was related to TL, and therefore, participants who accumulated more steps had longer leukocyte TL than participants who accumulated fewer steps per day. A recent randomized controlled trial (RCT) that evaluated the effect of aerobic exercise (120 min per week) versus usual inactivity reported that exercise induced changes in TL, promoting telomere lengthening (61). A different result was obtained in an intervention study made by Sjögren et al., since no significant associations were found between changes in steps per day and changes in TL (62). Moreover, Friedenreich et al. (63) found no effect of aerobic exercise on telomere attrition.
A study that included 2,401 Caucasian twins, of which 2,152 were women, reported that TL was positively associated with increased physical activity, and the most active subjects had an increase of approximately 200 nucleotides in TL as compared to the least active subjects (14). The above study is similar to a cross-sectional study performed by Dankel et al. (64), in which the participants who had an active life had longer telomeres compared to sedentary individuals. However, this relationship was not found in overweight or obese participants who were active; therefore, the authors suggest that obesity can mitigate the positive effects of physical activity on TL. This is consistent with the study reported by Mason et al. (65) in postmenopausal women, in which the authors did not find changes in TL among women who were overweight or obese, nor did they found significant changes in TL from the effect of dietary weight loss and aerobic exercise for 12 months. Based on these results the authors suggest that exercise intensity or duration may not be enough to change TL. However, moderate physical activity can have a positive impact through a reduction in adiposity, and can also reduce inflammation levels and oxidative stress (66), factors that attenuate telomere attrition. The latter two studies contradict the findings mentioned above and reflect the importance of carrying out more intervention studies to assess the effect of physical activity on TL.
In studies conducted with cancer patients or cancer survivors, the effect of physical activity on TL maintenance seems to be similar. An RCT performed with overweight and obese breast cancer survivors examined the effect of a 6-month diet and exercise-induced weight loss intervention versus usual care on TL. The authors found changes in TL among women with breast cancer in stage 0-I; there was a 7% telomere lengthening in the intervention group compared to an 8% shortening in the usual care group (p = 0.01) (67). Another study conducted by Loprinzi and Loenneke (68) found an inverse association between leukocyte TL and all causes of mortality among men who engage in moderate-intensity exercise, which suggests that moderate exercise prevents telomere shortening and increases survival. Ennour Idrissi et al. (18) found that total and occupational physical activity were positively associated with longer TL in 162 premenopausal and postmenopausal women with breast cancer. Furthermore, in a study performed with breast cancer survivors by Garland et al. (69), participants with moderate to vigorous physical activity had a longer TL compared with sedentary women. Physical activity may protect individuals from aging-related diseases, acting as a regulator of the cellular aging process. In this avenue of research, it makes sense to use TL as a biomarker of breast cancer risk and, at the same time, as an indicator of lifestyle changes.
POSSIBLE MECHANISMS OF ACTION OF OXIDATIVE STRESS AND INFLAMMATION IMPLIED IN THE REDUCTION OF TELOMERE SHORTENING
TL is a major determinant of biological age and represents a measurable outcome of the additive repercussions of both inflammation and oxidative stress (70); however, the mechanisms are not fully understood. Telomeres shorten at a rate of 40-200 bp per division (71). The rate of telomere shortening by cell division is not an innate constant and changes from one cell to another, possibly from one cycle of division to the next, depending on oxidative stress and defensive antioxidants (12). Telomeres are rich in guanines, which are prone to be oxidized to 8-oxo-2′-deoxyguanosine and 2,6-diamino-4-oxo-5-formamidopyrimidine. Reactive oxygen species, especially hydroxyl radicals, produce single-strand breaks, either directly or as an intermediate step in the repair of oxidative base modifications. Therefore, telomeric DNA appears to be deficient in the repair of single-strand breaks, which may increase the sensitivity of telomeres to the accumulation of 8-oxo-2′-deoxyguanosine DNA strand breaks (13,72). In addition, oxidative stress directly triggers the activation of the transcription factor NFκB, which is the key regulator of the inflammatory process that regulates transcription for various molecules such as interleukins and TNF-α, and is involved in the modulation of telomerase activity (73).
A majority of the reviewed studies conclude that the possible effects of diet or physical activity on reduced telomere attrition is due to a decrease in oxidative stress and inflammation. Among the main mechanisms attributable to physical activity and telomeric shortening are improved REDOX balance, favoring an expression response in antioxidant proteins and DNA repairing enzymes, as well as a reduction of reactive oxygen species, and therefore CRP, IL-6 and TNF-α levels (72). Additionally, exercise training potentially facilitates TL maintenance through many molecular mechanisms, since TL is regulated by epigenetic modifications such as histone changes (methylation and acetylation) and DNA methylation (73). Exercise also acts as a stimulus for telomerase transcription or activity. The hypothetical signaling pathways posited by the authors suggest increased TERT gene transcription as a response to exercise (74).
Chilton et al. (75) first reported that acute exercise can lead to the transcriptional regulation of several main telomeric genes in immune cells. Their results show an upregulation of the key telomeric gene TERT mRNA, which plays an important molecular role in telomere maintenance since it is related to telomerase activity, and a downregulation of TERF2IP mRNA. The authors also reported that exercise regulates miRNAs, including miR-186 and miR-96, with the potential to control the downstream expression of genes involved in telomere homeostasis. It is interesting to note that the mechanisms involved may depend on the specific exercise modality (75), such as resistance training adaptability (76) and aerobic fitness levels (77).
On the other hand, a healthy diet that contains antioxidant and anti-inflammatory components, coupled with physical activity, leads to improved inflammation levels and decreased oxidative stress, mechanisms that are involved in telomere shortening. A study reported that a high adherence to MD can stimulate telomerase activity in mononuclear cells, either directly by the effect of some specific nutrients included in the diet or indirectly by the global effect of the diet on the modulation of inflammation and oxidative stress (15,70). Specifically, IL-6 and TNF-α have been associated with telomere shortening due to their promotion of cell renewal, replicative senescence, induction of oxidative stress, and inhibition or promotion of telomerase activity (11). Likewise, the levels of IL-6 and TNF-α have been associated with daily energy intake, diet carbohydrate/fat proportions, cereals, and meat intake (27). The impact of diet on TL can be by dietary components in the food groups, such as isoflavones from legumes and seaweeds, by antioxidants, and by folic acid, all of which play an important role in DNA methylation and integrity. Another example is fish, which contains vitamin D and has anti-inflammatory and antiproliferative properties that limit the turnover of cells, thus potentially reducing their telomere length attrition (27). Figure 1 shows the possible effects of lifestyle factors (diet and physical activity) and their underlying biological mechanisms that may cause changes in telomere length.

Figure 1. Potential influence of healthy diet and physical activity on the maintenance of telomere length. This scheme represents how individually and collectively a healthy diet and the performance of physical activity can modify the mechanisms involved in maintaining telomere length, such as increased antioxidants and DNA repair enzymes, decreases reactive oxygen species, and reduced pro-inflammatory cytokines, as well as in promoting the methylation or acetylation of histones and DNA methylation.
CONCLUSIONS, LIMITATIONS AND FUTURE RESEARCH
The studies that evaluated the potential association of diet and TL found that adherence to MD and consumption of antioxidants, fiber, vegetables, seeds, and walnuts are associated with greater TL. In contrast, high consumption of sugary beverages, processed meat, and proinflammatory diets was associated with telomere shortening. Therefore, a healthy diet rich in antioxidants, such as carotenoids and vitamins C and E, and in anti-inflammatory components, such as vitamins A and D, polyphenols, fiber, and omega-3 fatty acids, has a greater effect on the decline in the rate of telomere shortening. These dietary components, especially omega-3 fatty acids, influence the potential mechanisms that reduce telomere shortening (51,78,79) due to their anti-inflammatory and antioxidant properties, as suggested by the study by Kiecolt-Glaser et al. (52), in which the intake of omega-3 fatty acids caused a decrease in IL-6, and IL-6 was associated with telomere lengthening.
On the other hand, findings from all studies demonstrated that performing moderate physical activity is associated with a longer TL; only one study reported that obesity attenuates this relationship, which could be related to the inflammation levels usually associated with obesity. Following this hypothesis, it is possible to say that an increase in physical activity together with a healthy diet may reduce inflammation and oxidative stress levels, and consequently telomere shortening rate. By reducing telomere shortening, these potentially modifiable factors can indirectly contribute to lowering the risk of chronic degenerative diseases such as cancer, or to improving the survival rate of people who have had cancer. However, recent papers regarding the relationship between TL and cancer risk revealed a complex scenario with contradictory findings depending on different cancer types (80,81). Likewise, Weischer et al. (82) measured leukocyte TL in a prospective study of 47,102 Danish population who were followed for up to 20 years for cancer diagnosis and death, and the authors found that short telomere length is associated with reduced survival after cancer but not with cancer risk. Therefore, the role of the relationship between TL and cancer risk remains to be demonstrated, and it could be a focus for future research.
It is important to emphasize that most of the studies presented in this review, and that evaluate the effect of diet or physical activity on TL, are cross-sectional in design. Therefore, the associations that were found referred to risk or protective factors and not to causality, since the criterion of temporality is not met. It is suggested that clinical intervention studies should be carried out to evaluate the effect of changes in adherence to a healthy diet and the performance of long-term physical activity on TL, considering the duration and intensity of the exercise ultimately practiced. It is also recommended that repeated measurements of TL over time be included in the design. For future studies food cooking habits of food are worth considering in order to evaluate the relationship between deeply fried or highly roasted foods and telomere shortening or lengthening. It is also recommended that studies be carried out that evaluate the joint effect of diet and physical activity on TL, since there are few studies on the subject – indeed, we only found four studies on this subject (30,31,32,65).
To build a complete picture of the possible mechanisms involved in the impact of these changes on the rate of telomeric shortening, of those that can be modulated with a healthy lifestyle, it is important to include biomarkers of oxidative stress, since telomeres are highly sensitive to the damage produced by this type of stress (14). Likewise, it is important to measure telomerase activity, since lifestyle factors may also affect this enzyme. While considering that diet is important, future research is also needed to provide evidence of the effect of individual dietary components, which in turn are part of the whole diet, on telomere shortening. Based on these findings, dietary guidelines could be created so that the population may acquire a healthier type of diet and, at the same time, reduce the risk of suffering from NCDs related to cellular aging or the shortening of telomeres.














