INTRODUCTION
Chronic diseases are highly prevalent in developed countries and account for a large percentage of health care spending. The pathogenesis and course of most chronic diseases can affect a patient’s nutritional status.
Heart failure is closely associated with nutrition, and a vicious circle is often observed between both conditions. Nutrition can be altered in the natural history of the disease as follows:
– Nutritional status usually worsens during episodes of acute decompensation. In fact, the prevalence of any degree of malnutrition is high in patients hospitalized for decompensated heart failure. Depending on the method used for screening or assessment, up to 57 % of patients may be at risk of malnutrition (1 2 3 4 5-6).
– Malnutrition is common during the course of the disease, regardless of whether the patient is in a decompensation episode. After 3 years of follow-up, the SOLVD study revealed a cumulative incidence of cachexia > 35 % (7), which is a sign of poor prognosis, independently of age, functional class, and left ventricular ejection fraction. Prognosis varies with nutritional status. In the most poorly nourished patients mortality is greater, hospital stay longer, and readmissions more numerous (8 9 10-11).
The nutritional status of patients with heart failure is affected by several factors:
– Anorexia (which is an adverse effect of certain drugs), variations in diet, and the sensation of abdominal fullness in patients with ascites and hepatomegaly.
– New York Heart Association (NYHA) functional class: Accelerated catabolism and neurohumoral activation are associated with a poorer NYHA class (12,13).
– Multifactorial inflammatory status. In fact, heart failure has progressed from being understood as a disease affecting a single organ to being now considered a systemic disorder that affects the immune, neuroendocrine, and metabolic systems (14).
– Alteration of intestinal absorption owing to congestion of veins in the affected area, loss of nutrients through enteropathy, and loss of proteins (15 16-17).
– The as yet unclear role of diuretics in nutrition.
An appropriate diet is a key component of most programs for the care of patients with heart failure. These programs combine education, motivation, and close follow-up. When the patient has malnutrition or is at risk of malnutrition, a quantitative and qualitative modification of oral intake is necessary, and the need for specific complementary nutrition must be evaluated (18).
The few clinical trials that have been performed in patients with chronic heart failure undergoing nutritional interventions with energy supplements show an increase in weight, although the effects on function and quality of life varied widely among studies (19). It seems reasonable to perform clinical trials that assess patients with heart failure who have malnutrition or are at risk of malnutrition in order to determine whether nutritional interventions provide solutions to the complex association between heart failure and nutrition. With the exception of low-sodium diet and liquid restrictions, there are currently no recommendations on nutritional interventions for patients with heart failure (20).
The objective of the present study was to determine whether nutritional advice and nutritional supplements can improve the nutritional status of patients with chronic heart failure who either have overt malnutrition or are at risk for malnutrition.
MATERIAL AND METHODS
This was a randomized clinical trial on an intention-to-treat basis. The assessors were blinded to the group patients were allocated to. Patients were randomized 1: 1 to 2 groups:
– Intervention arm: A structured educational intervention was provided to the patient and his/her main caregiver. The intervention examined dietary habits and provided drinkable normal-protein, high-calorie dietary supplements to be taken over a period of 12 weeks.
– Control arm: This group received the standard treatment: no supplements and no structured educational intervention. The patients received information about dietary habits from the nursing team and then in the discharge report, although never under the same conditions as the intervention group (time spent on, and form of the intervention).
INCLUSION CRITERIA
Patients had to be ≥ 18 years old and hospitalized with a diagnosis of chronic heart failure. In addition, they had to be hemodynamically stable with an acceptable control of symptoms to the extent that they were likely to be discharged. Patients had to be in one of the following situations:
– Nonintentional loss of > 5 % of their body weight without fluid overload during the previous 6 months
– Risk of malnutrition calculated based on the Subjective Global Assessment (SGA)
– Risk of malnutrition according to the Mini Nutritional Assessment (MNA).
We previously studied the concordance between SGA and MNA in patients with heart failure. Our group obtained an acceptable kappa index (0.637) (26). The MNA did not prove to be a predictor of death (9). The use of the MNA as a second method of nutritional assessment was to investigate the subjective burden of the SGA.
In addition, patients had to fulfill the following conditions:
EXCLUSION CRITERIA
Diagnosis with active cancer, dementia or severe cognitive impairment, advanced kidney failure with renal replacement therapy, and simultaneous participation in another clinical trial.
SAMPLE SIZE
Sample size was calculated according to the hypothesis that when follow-up was complete, at least 40 % more patients in the intervention arm would be free from malnutrition when compared to the control arm (i.e., they would pass from SGA grade B or C to grade A). Given the lack of sufficient evidence, the hypothesis for the calculation was agreed upon by the investigators. A loss of 25 % during follow-up was estimated. Each arm comprised 33 patients, although more patients were eventually included to take make up for losses higher than 25 %.
POPULATION
Patients were recruited from the cardiology and internal medicine areas during the in-hospital period. All of them had a diagnosis compatible with acute decompensated chronic heart failure and presented Framingham criteria for congestive heart failure.
RANDOMIZATION
A random number table was generated for 80 patients in a 1: 1 ratio. Identically sized strips of paper were prepared with the word “Control” or “Intervention” . The strips were folded in half, placed into opaque envelopes, which were then sealed. Numbers corresponding to the inclusion number were written on the outside of the envelope. The table generated by the randomization program was then destroyed. The envelopes were kept under lock and key.
OUTCOME MEASURES
Risk of malnutrition according to the SGA and MNA; functional capacity measured using the 6-minute walk test; and quality of life measured using the heart failure-specific quality of life questionnaire Minnesota Living with Heart Failure (MLHF) (27) adapted for use in Spain (28).
VARIABLES
In addition to the outcome measures we collected anthropometric data, sociodemographic data, clinical data, treatment data, and analytical data.
INTERVENTION
Patients were randomized to one of the groups once it was clear that they understood and agreed to participate in the study, and gave their written informed consent.
Patients randomized to the intervention group received a 75-minute individualized training session on nutrition with their main caregiver. They were shown strategies to improve their appetite, replace salt, prepare and serve food so that it was more appetizing, perform physical exercise adapted to their functional class, remember to take their medication, and take and store their dietary supplements appropriately.
They were also given a 12-week supply of normal-protein, high-calorie nutritional supplements (1 or 2 per day depending on intake); adherence was reported by the patient him/herself or by the main caregiver. Three different beverages were provided, depending on whether the patient had diabetes, chronical kidney failure (no dialysis patients were randomized), or neither of these comorbidities. Patients were informed that the nutritional shakes did not replace but complemented their diet, especially during periods of major anorexia. The control group received treatment as usual.
FOLLOW-UP
At 3 and at 9 months the assessor (who was blinded) recorded the nutritional status based on the SGA and MNA questionnaires, the result of the 6-minute walk test, the replies to the MLHF questionnaire, anthropometric measurements, and laboratory results. Assessors were trained in the administration of the SGA, and the degree of inter-observer agreement was assessed using the kappa index (a minimum value of 0.71 was considered indicative of good concordance).
STATISTICAL ANALYSIS
Normality was explored using the Kolmogorov-Smirnov test. Non-normally distributed variables for which the group was fewer than 35 individuals were expressed as median (IQR). Association between qualitative variables was evaluated using contingency tables and the chi-square or Fisher’s exact test. Quantitative variables were compared using the t-test. Statistical significance was set at p < 0.05 (Table I).
Table I. Quantitative variables, in different visits. p-values between the control and intervention group in each visit are at the end in italics
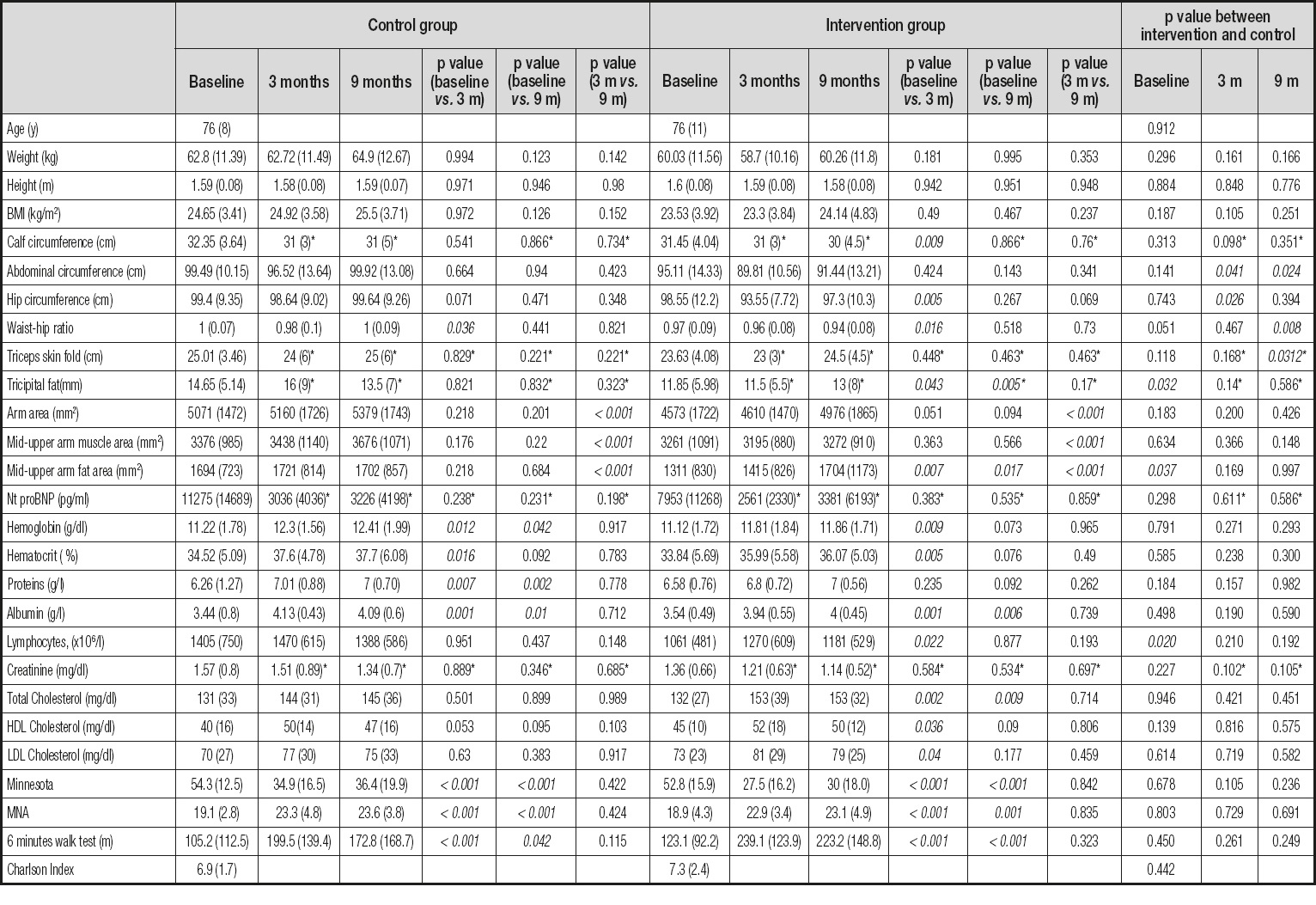
*Marked values do not present a normal distribution. They have been expressed with the median and the interquartile range and the p-value comes from non-parametric tests.
Nutritional status (MNA) and quality of life were evaluated by comparing the means for paired data. Non-normally distributed variables and cases where the groups had less than 35 members were analyzed using nonparametric tests. Significance was set at 5 % for all tests of acceptance or rejection of the null hypothesis.
Data were analyzed using the SPSS v. 21.0 software package.
RESULTS
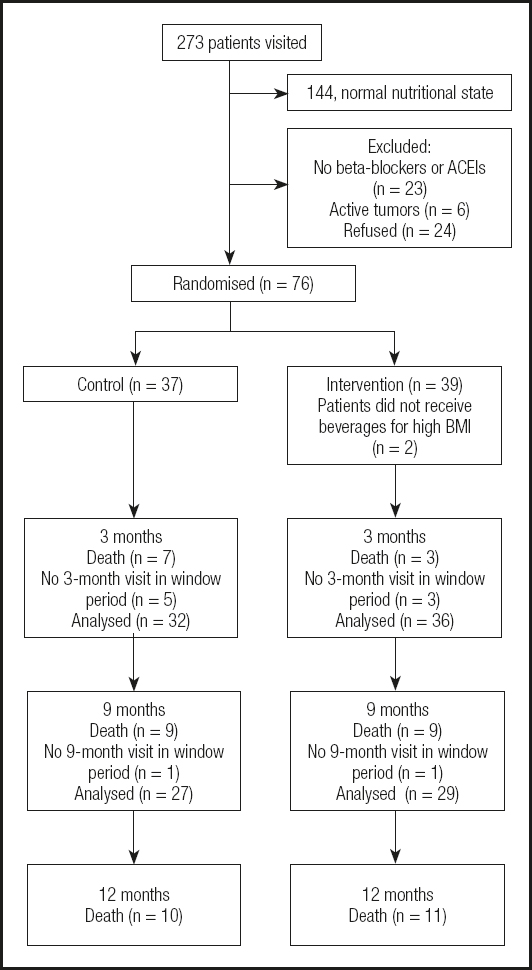
Patients admitted to the cardiology and internal medicine units with a diagnosis of decompensated heart failure were recruited from a total of 273 visits. The final sample comprised 76 patients.
The average profile of participants was that of a 70-year-old patient with a high Charlson comorbidity index and whose body mass index was not necessarily low. Mean weight was 61.3 ± 11.4 kg, with a body mass index of 24.0 ± 3.7 kg/m2.
Almost all of those who lived at home (69 patients, 90.8 %) ate food that was prepared at home either by themselves or by those who lived with them. The rest ate in day care centers or other services.
The distribution by gender was well balanced (52 % women). According to the SGA, 22.4 % of patients had malnutrition and 77.6 % were at risk of malnutrition. At least one-third did not have leg edema at the initial visit after intense diuretic treatment. Their NYHA functional class was II or III in 85 % of cases, that is, a moderate-to-severe reduction in their ability to perform daily activities. At the initial visit significant differences were detected in the mean values for tricipital skinfold (p = 0.032), arm fat area (p = 0.037), and lymphocyte count (p = 0.02). Lower values were found in the test group in all cases. Differences were also found in the SGA (p = 0.004), and nutritional status was poorer in the test group, since this was the group in which more individuals had overt malnutrition (35.9 % vs. 8.1 %).
The characteristics of these groups may be seen in the tables.
COMPARISON OF BOTH GROUPS AT MONTH 3
Patients from both arms died during this period, and many patients were considered to have had a normal nutritional status. Eight patients did not attend the 3-month follow-up visit within the expected interval (5 in the control group and 3 in the test group). The only differences found from baseline were in abdominal circumference and waist circumference – for both parameters the lowest values were detected in the test group. This difference was attributed to reduced edema. The evaluation of nutrition revealed no differences between the groups at month 3 neither in the MNA nor in the SGA. Of note, a difference was found at the baseline visit, where the test group was the least favored group in nutritional terms.
COMPARISON BETWEEN THE BASELINE AND 3-MONTH VISITS
The comparison of the baseline and 3-month visits for the control group revealed differences in the result of the 6-minute walk test (mean increase of 85 m), quality of life, MNA score, and hemoglobin, hematocrit, total protein, and albumin values. The mean values of all of these variables increased. The only variable for which a significant decrease was observed was the waist-hip index. This finding could be interpreted as a reduction in ascites. Despite the changes seen in nutritional classification according to the SGA, overall the results were similar at both visits, since the number of patients whose classification worsened was similar to that of patients whose classification improved.
The comparison of the baseline and 3-month visits for the test group revealed differences in abdominal circumference, waist-hip index, and calf circumference, all of which decreased. This finding can be interpreted as an improvement in edema and ascites. Statistically significant differences were detected in tricipital fat fold, arm fat area, quality of life, MNA score, hemoglobin, hematocrit, albumin, lymphocyte count, result of the 6-minute walk test (mean increase of 97 m), total cholesterol, HDL cholesterol, and LDL cholesterol. Of note, mean LDL cholesterol at this visit was 80.79 mg/dL, which was below the target for patients with ischemic heart disease.
The SGA showed that in the test group the nutritional classification improved 2-fold and worsened by half when compared with the control group; i.e., the total magnitude of the change was 4 times greater. The difference was statistically significant (Table II).
Table II. The table shows the Wilcoxon signed-rank test about SGA between the three-month visit and the nine-month visit vs. baseline-visit. The intervention group improved four times and twice with respect the baseline-visit. No changes were seen in the control group
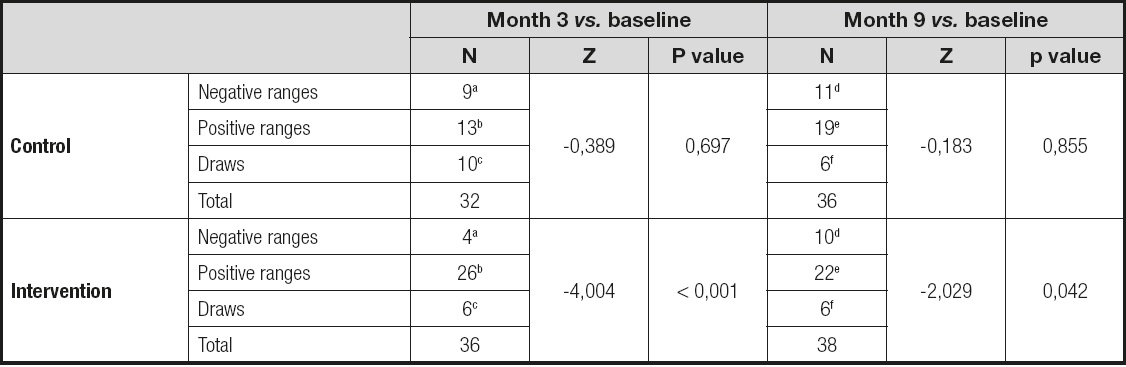
aSGA month 3 < SGA baseline;
bSGA month 3 > SGA baseline;
cSGA month 3 = SGA baseline;
dSGA month 9 < SGA baseline;
eSGA month 9 > SGA baseline;
fSGA month 9 = SGA baseline.
COMPARISON OF BOTH GROUPS AT MONTH 9
At this visit, statistically significant differences were found in abdominal circumference and waist-hip index. The lowest values were found in the test group, and were interpreted as a change in body fluid content. The number of patients who died in each group increased, thus reducing the sample size.
COMPARISON BETWEEN THE BASELINE AND 9-MONTH VISITS
The comparison of the baseline and 9-month visits in the control group revealed statistically significant differences in hemoglobin (mean increase of 1.2 g), total protein (g), albumin (g), MLHF score (mean decrease of 18 points: i.e., improved quality of life), MNA score, and result of the 6-minute walk test (mean increase of 67 m).
The comparison of the baseline and 9-month visits for the test group revealed statistically significant differences in the same parameters as the control group (except for total protein). Statistically significant differences were also found in parameters associated with energy reserve, namely, tricipital fat fold, arm fat area, and total cholesterol. The magnitude of the change in functional capacity assessed using the 6-minute walk test (mean increase of 100 m) and in quality of life (mean decrease of 22 points) was greater in the test group.
According to the SGA, compared with the control group the nutritional classification of the test group improved 2-fold between the baseline and 9-month visits, although no improvement was observed for the control group. The improvement seen in the test group was statistically significant.
COMPARISON BETWEEN THE 3-MONTH AND 9-MONTH VISITS
The variables for which statistically significant differences were recorded were — in both groups — total arm area, arm muscle area, and arm fat area. Total arm area increased in both groups, although arm fat area (energy reserve) increased by approximately 20 % in the test group; in the control group, it decreased between these two visits.
STUDY OF SURVIVAL
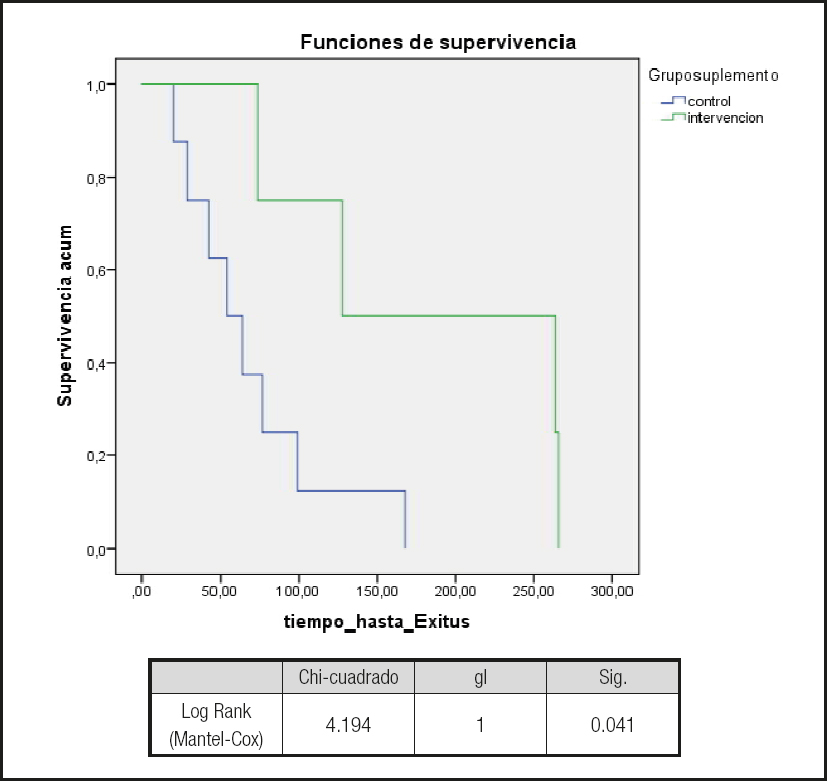
Overall, no differences were observed between groups in crude mortality at 1 year or in survival (Kaplan-Meier) (Fig. 2). In contrast, when only the patients who were considered to be at “nutritional risk” were selected and those patients who were considered to be “malnourhised” , according to the SGA in the baseline visit, were excluded, survival was greater in the patients with “nutritional risk” who were randomized to the test group (log-rank, 4.19; p = 0.041) (Fig. 3). No significant differences were observed between the test and control groups in this subpopulation (i.e., patients considered as being at nutritional risk in the SGA at the initial visit) for any of the variables (e.g., weight, body mass index, and laboratory values). Similarly, no differences were observed in the Charlson comorbidity index (control group, 6.9 vs. test group, 7.3; p = 0.383). This difference in mortality was lost if the MNA was used to evaluate nutrition.
DISCUSSION
Despite the fact that malnutrition in patients with heart failure is multifactorial, and that there is reasonable doubt about whether nutritional interventions are sufficient to break the vicious circle of malnutrition-disease, the results of this study and of other similar studies indicate that these patients can benefit from nutritional interventions. In fact, timely training bundles (22) and nutritional interventions have been shown to have a beneficial effect on nutritional status in critically ill patients (23,24). It seems logical to think that the broader the approach, the more chances of success the intervention will have. Along these lines, a Cochrane review showed that double nutritional interventions (dietary advice combined with supplements) are more efficacious than either of the two alone or no intervention, especially in patients with a BMI < 20 kg/m2 (19). Our study showed an improvement in nutritional status and an increase in the variables associated with energy reserve, which was greater in the test group after an educational-motivational intervention and after 12 weeks of nutritional supplementation.
The results of our survival analysis are interesting and consistent –except in very specific areas– with those of the PICNIC study (20,25) (a clinical trial sponsored by the Spanish Society of Cardiology involving patients hospitalized with heart failure, and a sufficiently large sample size to detect changes in mortality). In our study we detected differences in survival among patients classified by the SGA as at-risk during the baseline visit, although there were no differences in survival for those who were initially classed as malnourished, thus suggesting that nutritional interventions are more effective and efficient the earlier they start.
Unlike the PICNIC study (26), we were unable to demonstrate fewer readmissions, visits to the emergency department, and days of stay during follow-up over 1 year, since the sample size was calculated based on an improvement in nutritional status. In our case, it seems likely that the lack of homogeneity between groups at the baseline visit with respect to nutritional classification as based on the SGA tool made it impossible to demonstrate differences for these variables.
The PICNIC study differs from ours in important ways that could account for the differences in results as follows:
– The setting. We only included patients with exacerbations of chronic heart failure, which –we assume– increased mortality in our study.
– Inclusion criteria. As we also used the MNA, our inclusion criteria were different, and we observed greater prognostic sensitivity in the SGA1, perhaps because the experienced observer brings added sensitivity to the test.
In any case, the management of chronic diseases is changing. Patients with heart failure in particular may benefit from multidisciplinary programs based on integrated care, which they can access quickly and on demand as a result of functional class impairment. In addition to enhancing quality of life, these programs decrease the number of admissions for decompensation and mortality (27 28 29 30 31 32-33). Indeed, treatment is conceived not only as adherence to drug therapy, which should be appropriately monitored, but also as a suitable and healthy lifestyle. In addition to following guidelines on drug therapy, heart failure should be treated with a number of lifestyle interventions (aerobic exercise adapted to functional class), diet (low-sodium, antioxidant-rich diet), avoidance of harmful substances (smoking), optimization of treatment, and control of other risk factors (lipids, hypertension). Such is the origin of programs that –both in the hospital (34) and after discharge (18 19 20 21 22-23)– are aimed at maintaining quality of life and functional capacity, and preventing admissions to hospital where possible. Admissions are expensive, have both a direct and an indirect effect on the patient’s quality of life, and, in many cases, lead to a lasting reduction in the ability to carry out the activities of daily living.
Their high level of supporting evidence has resulted in these programs being recommended by scientific societies such as the European Society of Cardiology (35). The nursing service plays a key role in most cases. In heart failure, multidisciplinary follow-up programs in which the patient can access care quickly and on demand have proved cost-effective, since they reduce the number of days in hospital and of visits to the emergency department, and improve the patient’s quality of life even though, in some specific cases, there is no evidence of reduced mortality (36 37 38 39-40).
We would like to see nutritional interventions included in the care package for patients with heart failure, especially for those who are at risk of malnutrition. In this study we have tried to advance the extant knowledge concerning malnutrition and heart failure in a practical and accessible way, and have proposed potential solutions to daily issues such as loss of appetite, dysgeusia, and low-sodium diets. Every effort was made to enhance motivation, and the patient was made the center of his/her own access to care (empowerment). Moreover, the intervention targeted a very vulnerable group of patients whose prognosis seemed poor.
These interventions should be continued over time. It is not enough that a patient be cared for. Part of the success of these programs is based on the fact that the patient “feels” that he/she is being cared for. In our study, the most active part of the follow-up period was that spanning the first 12 weeks; the remaining period was restricted to monitoring the study variables. The motivational effect on behavior may be limited over time if it is not reinforced. In this sense, our results overlap with those of Harland et al. (41), who performed a clinical trial to evaluate methods for promoting physical activity. A meta-analysis on motivational interventions revealed that short interventions of even 15 minutes can prove effective, although the probability of success improves with the number of contacts with the patient and with prolonged follow-up periods (42).
Although training and motivational interventions alone cannot provide the ideal solution, there is solid evidence in favor of their inclusion in health care interventions (43).
Unfortunately, randomization returned non-homogeneous groups with respect to the SGA tool, one of the study’s endpoints. This has been an important limitation. Thus, we could not compare the final results of both groups. Instead, we verified that the evolution of subjects with respect to themselves was different in each group. The intervention group maintained their weight while reducing their abdominal circumference, which has been interpreted as a reduction in ascites. Not so in the control group. In the intervention group, too, all fatty folds and total cholesterol increased, which has been interpreted as an improvement in energy reserves.
LIMITATIONS
We do not know what is the specific weight of each part of the intervention. For example, we do not know how much of the improvement obtained in nutrition is due to the supplements administered, or to what extent increased knowledge and motivational content really modified habits.
The fact that the nutritional classification of the study groups, according to the SGA, was not homogeneous at baseline may have masked the development of variables such as hospitalization or mortality. It is important to remember that no differences in nutritional classification as based on the MNA questionnaire were found between groups during the baseline visit (see table).
CONCLUSIONS
We found that advice on nutrition and adapted physical exercise accompanied by high-calorie, normal-protein supplements for 12 weeks improved nutritional status at 3 and 9 months (as measured using the SGA) in patients with chronic heart failure taking ACE inhibitors/ARA II or betablockers.
In patients with chronic heart failure at risk of malnutrition according to the SGA, who received a double nutritional intervention (advice plus supplements), survival was better than in patients with the same nutritional status who did not receive the intervention. This increase in survival was not observed in patients who already had overt malnutrition. It is important to remember that sample size was not calculated to distinguish changes in mortality.