INTRODUCTION
Human milk oligosaccharides (HMOs) are found in abundance in human milk and make up the largest solid component after lactose and lipids (1-4). Bovine milk, in contrast to human milk, contains relatively low levels of oligosaccharides, and the prevalence of fucosylated oligosaccharides, in particular, is quite low (5). 2’fucosyllactose (2’FL) is a trisaccharide composed of glucose, galactose, and fucose, and is one of the most abundant HMOs. Levels of 2’FL vary depending on the secretor blood group status of an individual woman as well as on ethnicity and stage of lactation, with 2’FL levels from about 0.9 to above 4 gram/liter (g/L) in mature milk among secretors (6-14). Another HMO in human milk is lacto-N-neotetraose (LNnT), in levels ranging from 0.1 to 0.6 g/L, with higher levels within the first month of lactation (7-10,15-17).
Evidence is emerging that HMOs play an important role in the development of a balanced intestinal microbiota and in supporting immune protection in breastfed infants (18-20). Preclinical models have found that both 2’FL and LNnT promote the growth of Bifidobacterium species (21,22). Additionally, in a randomized controlled trial (RCT) of a term infant formula supplemented with 2’FL and LNnT, lower rates of parent-reported morbidity (particularly lower respiratory tract illnesses such as bronchitis) and lower use of antipyretics and antibiotics in the group receiving HMO-supplemented formula were reported as compared to the control fed infants (23). In a subset of the infants in this same RCT, stool samples collected for microbiota assessment and metabolic signature at three months showed that the addition of 2’FL and LNnT shifted the stool microbiota closer to that observed in breastfed infants both in composition and function (24). Collectively, these findings, in conjunction with the documented differences in HMO composition between human and bovine milk, have provided a solid rationale for supplementing bovine milk-based infant formulas with HMOs.
Advancements in manufacturing technology now enable the synthesis of HMOs, and preclinical studies have established their safety for the purposes of supplementation of infant formulas (25,26). Safety, tolerance, and adequate growth, as well as potential clinical benefits, have been demonstrated in RCTs of term infant formulas supplemented with 2’FL alone and in combination with LNnT (23,27,28). The first such report was an RCT of growth and tolerance conducted in the US, which found that infants receiving a formula supplemented with either galacto-oligosaccharides [GOS] or GOS + 2’FL demonstrated adequate growth and good tolerance (27). A second RCT conducted in Belgium and Italy examined a study formula containing 1.0 g/L of 2’FL and 0.5 g/L of LNnT in the test arms, while the control arm received a standard formula without HMOs (23). The HMO-supplemented formula was again well-tolerated and supported age-appropriate growth. A third study in the US compared tolerance in infants receiving a 100 % whey, partially hydrolyzed infant formula with the probiotic B. lactis, with and without the further addition of 2’FL, and found that the HMO-supplemented formula was well tolerated (28).
While the evidence provided to date in RCTs is supportive of the safety and tolerance of HMO-supplemented infant formulas, studies are needed in a real-world setting because results from a highly controlled RCT do not always translate outside of the trial setting (29). Additionally, a relatively large proportion of infants in real-world settings are fed both human milk and formula (30-32), a mixed feeding regimen not studied in previous RCTs. The current study was thus designed to complement and enhance existing RCTs by assessing the growth, safety and tolerance of healthy term infants consuming an infant formula supplemented with HMOs either exclusively or mixed with human milk in a real-world setting.
PARTICIPANTS AND METHODS
STUDY DESIGN
This was a three-group, non-randomized, open-label, prospective study in healthy, term (37-42 weeks of gestation) infants enrolled at age 7 days to 2 months. The study was conducted between October 2018 and March 2019 in six centres throughout Spain (Quiron-Dexeus Universitary Hospital, Barcelona; Casa de Salud Hospital, Valencia; Maternal MH Belén Hospital, A Coruña; Paediatric Hispalense Group, Sevilla; Vitha Santa Catalina Hospital, Las Palmas; Ruber International Hospital, Madrid). One study group included infants who were exclusively formula fed (FF) while a second group included infants who were fed a mixture of formula and human milk (MF). The third group included exclusively breastfed infants (BF) serving as a reference population. Formula-fed infants were eligible to participate if their parent(s) had independently elected, before study enrolment, to formula feed. Breastfed infants were eligible if the infants had been exclusively breastfed since birth, and their parent(s) had decided to continue exclusively breastfeeding until at least four months of age. Exclusion criteria included any known intolerance/allergy to cow’s milk (formula-fed group only); conditions requiring infant feedings other than those specified in the protocol; evidence of significant systemic disorders (cardiac, respiratory, endocrinological, hematologic, gastrointestinal, or other); or parental refusal to participate.
At study enrolment, FF and MF infants received the study formula and continued to be fed the study formula for approximately 8 weeks (56 days). The formula was prepared and fed at home, and was given ad libitum. Infants completed an in-person clinic visit at enrollment (baseline) and again at day 56 ± 3 days (week 8 visit). A telephone visit with the parents was also conducted on day 28 ± 3 days (week 4 visit).
STUDY PRODUCT
The study formula was provided to the participants. Commercially available in Spain since 2017, it was a partially hydrolyzed, 100 % whey, term infant reconstituted formula with 67 kcal/100 mL consisting of 1.9 g of protein, 11.5 g of carbohydrates, and 5.1 g of lipids per 100 kcal powder, and with two HMOs: 1.0 g/L of 2’FL and 0.5 g/L of LNnT. The formula also included Lactobacillus reuteri (DSM 17938), vitamins and minerals.
ETHICAL APPROVAL AND INFORMED CONSENT
This study protocol was approved by the coordinating Hospital Institutional Review Board of Puerta de Hierro, Majadahonda (Madrid). Prior to the conduct of any screening tests, an informed consent was obtained from each participant’s parent. Good clinical practice was followed by all sites throughout the study. The study was registered with ClinicalTrials.gov (NCT04055363).
STUDY MEASURES
At baseline, anthropometry measures were obtained including weight, length, and head circumference using standardized procedures. Anthropometric parameters were measured again during the clinic visit at week 8. BMI was calculated as weight (kg)/length (m)2. Z-scores for weight-for-age, length-for-age, head circumference-for-age, and BMI-for-age were calculated using the WHO Child Growth Standards (33).
The infant’s gastrointestinal (GI) symptom burden was assessed via the Infant Gastrointestinal Symptom Questionnaire (IGSQ), a validated 13-item questionnaire that assesses GI-related signs and symptoms as observed by parents over the previous week in 5 domains: stooling, spitting up/vomiting, gassiness, crying, and fussing. Each item is scored on a scale of 1 to 5 with higher values indicating greater GI distress. A composite IGSQ score is derived from summing the individual scores with a possible range of 13 to 65, where higher values indicate greater GI distress and values ≤ 23 indicate no digestive distress (34). The IGSQ was administered at baseline, week 4, and week 8.
A formula satisfaction questionnaire was administered to parents of infants in the formula-fed groups at week 4 and week 8 including three questions regarding the parents’ experience with the study formula. Questions included ‘Did your child like what he/she consumed?’, ‘How satisfied are you overall with the study product?’, and ‘Would you continue to provide the study formula to your child?’
Adverse Events (AE) were captured from the time of enrollment through the end of study. All AEs were assessed by the site investigator for duration, intensity, frequency, and relationship to study formula. AEs were classified into System, Organ, and Class (MedDRA SOC codes).
STATISTICAL METHODS
Demographics and other baseline characteristics were compared for overall differences between the feeding groups using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. Pairwise comparisons were done using two-sided Wilcoxon’s rank-sum tests (continuous variables) and two-sided Fisher’s exact tests (categorical variables) adjusted for multiplicity by the Holm procedure. Fisher’s exact tests were computed from contingency tables. For tables larger than 2 x 2 a Monte Carlo estimation of the exact p-value was performed with 20,000 samples, otherwise a direct exact p-value computation was performed. Missing values were excluded before performing the aforementioned tests.
The co-primary outcomes were growth and composite IGSQ score. Feeding group comparisons were assessed for all growth measures using the analysis of covariance (ANCOVA) with adjustment for baseline value, age, gender, and study center. Tolerance was assessed via the IGSQ scores. The 13 individual questions in the IGSQ, as well as the five domain scores, were tabulated for each feeding group at each time point. The composite IGSQ scores were calculated for each feeding group and compared between feeding groups using ANCOVA with adjustment for baseline IGSQ score.
All analyses were conducted using the SAS software, version 9.3 (SAS Institute Inc., Cary, NC, USA). Statistical significance was assessed using an α level of 5 % with a two-sided test.
Being a RWE study, sample size was determined based on previous RCTs, aiming to have an analysis set of at least 40-50 subjects per group. Infants who were not compliant with the study protocol were withdrawn from the study and did not continue to participate in the study measurements or visits. The analysis set was defined by excluding infants who did not have a growth and/or tolerance measurement at week 8. All analyses of growth, tolerance, and satisfaction in this paper were conducted in the analysis set. Adverse events were reported for all enrolled infants.
RESULTS
SUBJECT DISPOSITION AND DEMOGRAPHICS
In this study, 207 infants were enrolled including 82 exclusively formula-fed (FF), 62 mixed-fed (MF), and 63 exclusively breastfed (BF) infants (Fig. 1). The number of subjects in the analysis set (those with tolerance and growth measurements at the end of 8 weeks of study) were 66, 48, and 45, respectively, in the FF, MF, and BF groups, with primary exclusion reasons being major protocol deviations and losses to follow-up.
The demographics and baseline characteristics of all enrolled infants are shown in table I. The FF group was slightly younger at enrollment as compared with the MF group and BF group (p < 0.01), with more male infants in contrast to the MF or BF groups. Gender distribution was, however, not statistically different between groups (p > 0.05). Other baseline characteristics were also comparable across the feeding groups, including age, educational attainment, and smoking status of the mothers and fathers. The baseline characteristics of the analysis set (n = 159) were similar to those of all enrolled infants for all feeding groups (data not shown).
Table I. Demographics and baseline characteristics (shown as N and % unless otherwise noted), by feeding group, all enrolled subjects (n = 207)

BF: exclusively breastfed; MF: mixed fed; FF: formula fed (exclusively); IQR: interquartile range (Q1-Q3); SD: standard deviation. 1Global p-values to detect any difference between the three feeding groups using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. 2FF different from MF and BF (< 0.01) using two-sided Wilcoxon rank-sum tests adjusted for multiplicity by the Holm procedure. 3BF different from MF and FF (< 0.01) using two-sided Wilcoxon rank-sum tests adjusted for multiplicity by the Holm procedure.
GROWTH
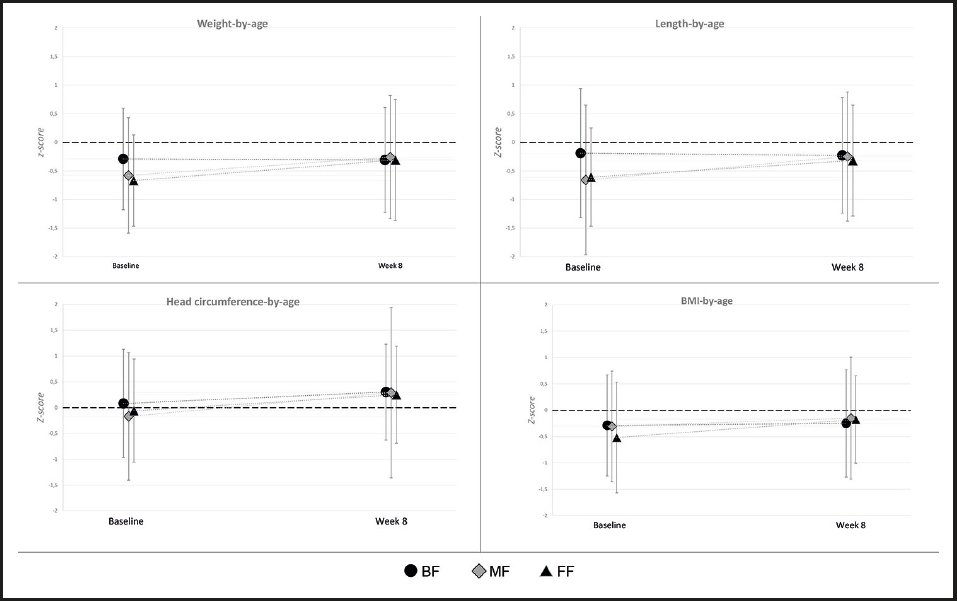
Overall, age-appropriate growth was observed in all three feeding groups. Baseline weight and length were slightly lower in the FF group (FF mean weight of 3.34 kg versus 3.75 and 3.93 in the MF and BF groups, respectively; FF mean length of 50.62 cm versus 51.86 and 52.82 cm in the MF and BF groups, respectively), which is consistent with the slightly younger age. By Week 8, there were no significant differences between any feeding groups for any of the anthropometric measures (all ANCOVA p-values between feeding groups > 0.05). Mean Z-scores for weight, length, head circumference, and BMI at baseline and week 8 are shown in figure 2. Weight-for-age, length-for-age, and BMI-for-age z-scores were similar between all feeding groups, and the mean z-scores were within ± 0.5 of the WHO medians at week 8. Head circumference-for-age z-scores were also similar between groups and tracked closely with the WHO standards.
GASTROINTESTINAL TOLERANCE
Table II shows descriptive characteristics for the five IGSQ domains and the overall composite score. Composite IGSQ scores demonstrated low GI distress in all feeding groups at all time points, and there were no significant differences between feeding groups at baseline, week 4, or week 8. Within each feeding group, for four of the five domains of the IGSQ (gassiness, fussiness, crying, and spitting-up/vomiting) there were no significant differences in scores between baseline and week 4 or week 8 (data not shown). For the fifth domain, stooling, FF infants had scores significantly different from the ones in the BF group (p < 0.001) at baseline, and showed significant improvement at week 8 (mean change [95 % confidence interval] = -0.79 [-1.35, -0.23], p < 0.01), moving closer to the stooling profile of the BF group. The scores for stooling of MF infants were significantly different from those of the BF infants at baseline (p < 0.05) and week 8 (p < 0.05).
FORMULA SATISFACTION
Formula satisfaction is summarized in table III. Nearly all parents reported satisfaction with the study product. More than 90 % of parents also reported at both week 4 and week 8 that their child liked what he/she consumed and that they would continue to provide the study formula to their child.
ADVERSE EVENTS
A total of 49 subjects experienced 58 adverse events (AEs) during the course of the study, including 18 AEs in the BF group, 21 in the MF group, and 19 in the FF group. Three patients experienced potentially product-related AEs, including two instances of cow’s milk intolerance, one in the FF group and one in the MF group, and one instance of irritability in the FF group. Six serious adverse events occurred (4 in the FF group and 2 in the MF group), all of which were bronchiolitis, and all were considered unrelated to the study feeding. The incidence of AEs was low overall and was not significantly different in the three feeding groups.
DISCUSSION
This is the first RWE in infants fed a formula supplemented with the HMOs 2’FL and LNnT, and containing L. reuteri, in which a breastfed and a mixed-fed group are included. The results demonstrate that formula-fed infants, either exclusively or mixed fed, receiving the HMO-supplemented formula had age-appropriate growth in line with the WHO standards, and that growth was also comparable to that seen in previous studies with west and south European infant populations (35). The formula was well tolerated, as indicated by low IGSQ scores, and GI tolerance in the formula-fed infants was comparable to that in breastfed infants. There was also a low incidence of adverse events in all groups, and despite of the season of the year (fall-winter), cases of bronchiolitis were lower than expected from the literature (36). Finally, parents provided high satisfaction ratings for the HMO-supplemented formula.
The results of this RWE study are similar to those from previous RCTs that have also examined term infant formulas supplemented with HMOs. One RCT was a multicenter, double-blind trial that enrolled 175 healthy term infants in Italy and Belgium at less than 14 days of age, who were fed a study formula for 6 months (23). The HMO-supplemented formula demonstrated age-appropriate growth as well as good tolerance as measured by parents. Another RCT included 189 term infants in the US who were randomized to 1 of 3 different formula groups (27). Infants were exclusively formula fed until 4 months of age. All formulas were considered well tolerated based on parental reports, and no significant differences were observed for growth between groups. Notably, neither of those trials utilized a validated tool to assess tolerance, and thus tolerance outcomes cannot be easily compared across studies. A recent RCT used the same validated IGSQ tool as in the current study to assess tolerance (28). The HMO-supplemented formula was well tolerated as evidenced by similar IGSQ scores between the groups with and without the addition of 2’FL. The agreements between the previous RCTs and the current study in a real-world setting, however, are reassuring that growth, safety and tolerance of the HMO-supplemented formula are consistent and robust across study designs.
This study included several strengths. First, GI burden was measured using a validated instrument, the 13-item IGSQ based on five separate domains of feeding tolerance. The use of a validated instrument provides information that is interpretable by and meaningful to practicing clinicians. Second, this was a RWE study, a design distinct from an RCT, simpler, less restrictive, and in line with current clinical practices, enhancing the generalizability of the results and providing additional useful information. The published prevalence of infants who are mixed fed, in Spain (30) and in the US (31) indicate that at age 1 month, 30 % of infants receive mixed feedings, similarly as in this study. Thus, the demonstration of appropriate growth and good tolerance effects in the mixed feeding group of infants in this study provides important evidence not found in the RCTs conducted to date.
Some limitations of the study should also be noted. An open-labeled, non-randomized design increases the risk for bias, in particular for response bias (also for validated questionnaires), and higher attrition rates and missing data. In a study with specifically defined feeding regimens such as ours, randomization is however not possible. The main aim of randomization is to have study groups with equal characteristics. We therefore compared the baseline characteristics in our three groups and there were no substantial differences except for age, which was lower in the FF as compared to the MF and BF groups. As we corrected all our statistical models with baseline age, we therefore assume that our outcomes are not significantly affected by the slight difference in baseline age between the groups, and hence also not by the non-randomized nature of our trial. The study formula was supplemented with just a single level of 2’FL and LNnT, and thus this study cannot assess whether the observed growth and tolerance effects might differ over a wider range of levels of these HMOs. Additionally, this study, while multi-center, took place within a single country (Spain) and its results may not be generalizable outside of western countries.
In conclusion, this is the first RWE study to examine the effectiveness of supplementing infant formula with HMOs. The results obtained were similar to those found in more tightly controlled RCT settings, indicating robust effects for growth, safety, and tolerance in association with HMO-supplemented infant formulas.