INTRODUCTION
In April 2002, the National Food Authority of Sweden published a study in which the presence of a carcinogen was reported for the first time in experimental animals, identified as acrylamide. The main mechanism for its formation in foods is through the Maillard reaction between reducing sugars (such as glucose or fructose) and the amino terminal region of asparagine, during the thermal process (T > 120 °C) (1).
In relation to health risks for the general population, limits of 1.0 to 4.0 μg/kg BW/day of oral exposure have been established for moderate to high consumption, respectively, and the safety limit to prevent neurotoxicity has been established below 200 μg/day. In countries such as Canada or China, the daily intake of acrylamide varies from 0.17 to 1.17 mg/kg BW/day, and this concentration depends on the dietary patterns of each population (2).
The Group on Contaminants in the Food Chain of the European Food Safety Authority (3) recently described the data of the presence of acrylamide in foods based on approximately 7,500 results reported by 24 countries, which combined demonstrate the exposure of the European population to this compound. Infants and children were the most exposed groups. Chronic dietary exposures of adolescents, adults, and the elderly were estimated between 0.3 and 0.9 μg/kg BW/day, and the 95th percentile between 0.6 and 2.0 μg/kg BW/day.
The mushroom Pleurotus ostreatus has been widely studied in relation to the antioxidant and antitumor effect of the β-glucans contained in its cell wall. The oyster mushroom or Pleurotus ostreatus is the easiest-to-grow edible mushroom variety, with excellent nutritional and medicinal properties. Since the production of fungi by traditional culture and the extraction of bioactive metabolites in some cases are very long and wasteful processes, biotechnology is fundamental for the development of profitable and productive techniques for obtaining these metabolites (4). A large number of bioactive compounds such as lectins, polysaccharides, polysaccharides-peptides, and polysaccharide-protein complexes have been isolated from this fungus, and it has been found that many of these compounds have hypocholesterolemic, antiviral, antibacterial, immunomodulatory, and anticancer effects (5).
METHODOLOGY
PLEUROTUS OSTREATUS FUNGUS SAMPLE
The fungus was obtained from the culture collection belonging to the Industrial Microbiology Laboratory. Mature fungi were washed perfectly well in distilled water, then dried and disinfected with ethanol (70 % v/v) before extracting the β-glucans present in the cell wall.
β-GLUCANS FROM PLEUROTUS OSTREATUS
β-glucans were obtained by the method described by Fleet and Manners (6), with some modifications made by Catley (7). After the harvest, the fungus was milled to fractionate the fruiting body, which contains the β-glucans. Briefly, 1 g of fungus extract (pulverized) was put in contact with 10 mL of NaOH (4 % w/v). The tubes were placed in a water bath at 80 °C for 4 hours. Subsequently, they were centrifuged at 5000 rpm for 20 min. The supernatant (alkali-soluble-glucan-mannan) was separated from the insoluble fraction-glucan-chitin (precipitate). The alkali-soluble fraction was separated from the insoluble fraction, recovered and added to 5 mL of CH3COONa (2M). Samples were kept at 75 °C in a water bath. Subsequently, 100 mL of ethanol (96 % v/v) were added and allowed to stand for 12 hours under refrigeration at 4 °C, after which time samples were centrifuged at 5000 rpm for 20 minutes, discarding the precipitate and recovering the supernatant, which contains the β-glucans.
The insoluble alkali material was re-extracted to recover the β-glucans, adding to the precipitate 5 mL of NaOH (6 % w/v) at 80 °C for 3 hours. Then the samples were centrifuged at 5000 rpm for 20 minutes, separating the sediment from the supernatant. The supernatant was treated with 5 mL of CH3COONa (2M), and incubated at 75 °C for 2 hours. Subsequently, 100 mL of ethanol (96 %) were added, allowing the samples to stand for 24 hours at 4 °C. The supernatants were pooled and passed to a dialysis membrane (Spectra/Pro, 12,000 to 14,000 MWCO). The samples were dialyzed at 4 °C for 24 hours with constant agitation at 70 rpm in a shaking incubator (Labtech), changing the solution of phosphate buffer (50 mM), pH = 7.4, plus NaCl (0.2 M) every 8 hours. Finally, the dialyzed concentrate was ultrafiltered with a membrane of 3 kilodaltons MW (Spectra Por), and the factions obtained were lyophilized and stored in freezing until their use.
INTAKE OF ACRYLAMIDE AND ADMINISTRATION OF β-GLUCANS
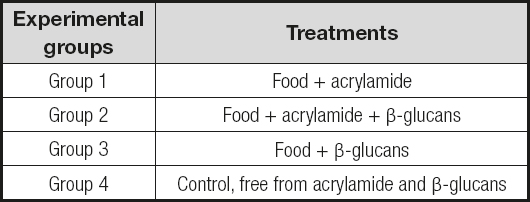
The experiment was performed with Balb/c male mice previously acclimated and housed in plastic cages; they were handled according to the Official Mexican Standard NOM-062-ZOO-1999. During the period of the experiment, the mice were kept under conditions of 12: 12 light/dark and a temperature of 25 °C, and were provided with water and feed ad libitum. For the evaluation of this part of the experiment, 4 groups of mice were made as depicted in table I. The amount of acrylamide and β-glucans was determined according to an estimate of the intake reported in other studies, and adjusted at concentration of 50 μg/kg BW for acrylamide and 50 mg/kg per day of β-glucan.
EVALUATION OF LIPID OXIDATION BY THE TBARS (THIOBARBITURIC ACID REACTIVE SUBSTANCES) METHOD
Lipid oxidation was evaluated by the formation of oxidation byproducts using the spectrophotometric TBARS method. The method was applied according to what was reported by Alamed et al. (8). The TBARS solution was prepared with trichloroacetic acid (15 % w/v) and thiobarbituric acid (0.375 % w/v) dissolved in HCl (0.25 M); subsequently, 3 mL of butylated hydroxytoluene (BHT) (2 % w/v) in ethanol was mixed with the previous solution. The TBARS reagent (2 mL), 0.050 mL of liver homogeinizate and 0.95 mL of water, was mixed in an amber vial using a vortex for 30 seconds; subsequently the mixture was placed in a water bath at 90 °C for 15 minutes. The vials were cooled to room temperature and centrifuged at 1000 x g for 15 min. The supernatant was placed in a cell, and the absorbance was measured in a UV-VIS spectrophotometer (Agilent 8453) at 532 nm. TBARS concentration was determined from a standard curve constructed by making appropriate dilutions of an aqueous solution of 1,1,3,3-tetraethoxypropane.
RESULTS
β-GLUCANS FROM PLEUROTUS OSTREATUS
β-glucans were obtained by alkaline extraction and subsequent ultrafiltration from the whole body of the mature and fresh fungus; 3.4 grams of extract equivalent to 6.3 % of whole fungus were obtained. This extract had a concentration of 80 % β-glucans, determined by HPLC and calculated with a calibration curve constructed with different concentrations of β-glucan.
GENERAL TOXICITY
No mortality was observed after exposure to the tested dose of acrylamide (50 μg/kg BW), only signs of peripheral neuropathy such as hyperactivity and tremors were observed after five days of experimentation, and they were maintained over 30 days after the experiment.
On the other hand, mice treated with acrylamide at a concentration of 50 μg/kg BW (group 1) showed an increase of 11.85 % in body weight with respect to the control animals (group 4). On the other hand, group 2, treated with β-glucans + acrylamide, showed no significant differences (p < 0.05) versus the control group. These results indicate that β-glucans may offer protection against acrylamide toxicity, and prevent problems associated with the obesogenic properties of this toxin. Table II shows the differences in weight between the mice of the different groups assessed.
EVALUATION OF LIPID OXIDATION IN MICE LIVER
The data obtained in our work indicate an increase in the levels of lipid peroxidation in the livers of acrylamide-treated mice, where an increase of almost 50 % was observed with respect to the control group (group 4). On the other hand, in group 2 (animals treated with acrylamide + β-glucans) the lowest levels of peroxidation were observed (0.00024 mM MDA); when compared to the control group, levels were even lower than in the group treated with glucans, which may be because acrylamide induced the activity of enzymes involved in the process of protection against oxidation, which could present a synergistic effect with β-glucans, and improve the antioxidant protection provided by these.
DISCUSSION
GENERAL TOXICITY
At the evaluated dose of acrylamide (50 μg/kg BW) no mortality was observed after exposure, only signs of peripheral neuropathy and body weight gain, since mice treated with acrylamide at a concentration of 50 μg/kg BW (group 1) showed an increase of 11.85 % in body weight with respect to the control group (group 4).
Studies have indicated that male rats administered acrylamide (5, 10 and 15 mg/kg/day) for eigth weeks had a significant decrease in body weight (9). On the other hand, daily exposure to acrylamide (5 mg/kg/day) led to a reduction in food-motivated behavior in adolescent rats (10). Moreover, the male rats fed with acrylamide (0.28 μg/kg BW), generated by frying oil for 12 weeks, a close dose to that of human daily exposure, also presented a slow rate of body weight growth (11). Conversely, in the present work, the group of mice treated with acrylamide at a concentration of 50 μg/kg BW (group 1) showed an increase of 11.85 % in body weight with respect to the control group (group 4). On the other hand, group 2, which was treated with β-glucans + acrylamide, showed no significant differences (p < 0.05) versus the control group. These results indicate that β-glucans may offer protection against the toxicity of acrylamide and prevent problems associated with the obesogenic properties of this toxin. These results are in agreement with results from previous epidemiologic studies, which hypothesized that long-term exposure to acrylamide might be associated with obesity-related outcomes, and that have indicated that small size at birth is a risk factor for a range of metabolic disorders, including a higher body mass index in adulthood, insulin resistance, increased visceral adiposity, and impaired glucose tolerance (12-14). Other results suggested that exposure to acrylamide disrupt metabolic homeostasis and subsequently lead to obesity-related disorders. As an electrophilic molecule, acrylamide is capable of reacting directly with sulfhydryl groups and amino residues of enzymes, receptors, and cytoskeletal proteins via its affinity for their nucleophilic sites; thus, acrylamide may affect a multitude of cellular processes, which has been proposed to formulate the basis of some of its toxic effects (15), and contribute to the causations observed in our current work. Secondly, gut microbial ecology also impacts on the metabolism and biotransformation of environmental toxins, and has been linked with the etiology of obesity. Exposure to environmental chemicals at doses comparable to those estimated for human exposure is capable of modifying the composition of the gut microbiota in rats (16).
EVALUATION OF LIPID OXIDATION IN MICE LIVER
It has been reported that acrylamide is a cause of lipid, protein, and nucleic acid oxidation, and of activation of apoptotic pathways (17). Studies have revealed a significant increase in the level of malondialdehyde (MDA) in the serum, liver, kidneys, testes, and brain of rats treated with acrylamide. According to Motamedshariaty et al. (18), MDA is one of the main oxidation products of peroxidized polyunsaturated fatty acids, and therefore an increase in MDA content is an important indicator of lipid peroxidation; its occurrence in biological membranes causes their deterioration and the inactivation of several membrane-linked enzymes (19,20). On the other hand, Lakshmi et al. (21) revealed that acrylamide increases the levels of lipid peroxidation products, such as the content of protein carbonyls and hydroxyl radicals. Altinoz et al. (22) showed that the administration of acrylamide increased MDA levels in the liver and tissues of the small and large intestine. The data obtained in our work indicate an increase in the levels of lipid peroxidation in the livers of acrylamide-treated mice, where an increase of almost 50 % was observed with respect to the control group (group 4). On the other hand, in group 2 (rats treated with acrylamide + β-glucans), the lowest levels of peroxidation were observed (0.00024 mM MDA). Compared to the control group, these levels were even lower than in the group treated with glucans, which may be because acrylamide induced the activity of enzymes involved in the process of protection against oxidation, which may present a synergistic effect with β-glucans and improve the antioxidant protection provided by these. Our results support the clinical use of β-glucans to treat oxidative stress, a condition that may cause organ failure. The administration of β-glucans may provide protection against oxidation caused by acrylamide. A dose of 3.5 mg of β-glucans in a 70 kg person would be necessary to significantly reduce the deleterious effects caused by acrylamide ingestion. However, further studies remain necessary.
CONCLUSIONS
The results show that β-glucans may act as antioxidant agents, and may protect the liver against the oxidative stress caused by the acrylamide ingestion.
Our results support the clinical use of β-glucans to treat oxidative stress, a condition that may cause organ failure. However, more studies are needed to assess the effectivity, security, and dosage of these compounds as treatment—based in the fungus Pleurotus ostreatus—against acrylamide.