INTRODUCTION
Obesity is a public health problem worldwide. In 2016, more than 1.9 billion adults, 18 years and older, were overweight. Of these, over 650 million were obese (1). Mexico presents a high prevalence of obesity. The ENSANUT MC, 2016 reported that the prevalence of abdominal obesity was 76.6 %, this being higher in women than in men (87.7 % vs. 65.4 %) and in the group of 40- to 79-year-olders as compared with the group of subjects aged 20 to 29 years (2). According to Rtveladze et al., by 2050 in Mexico, there will be a greater population with obesity, as well as a considerable increase in cases of diabetes and cardiovascular diseases (3). Obesity is often associated with insulin resistance and the metabolic syndrome. Metabolic syndrome (MetS) is characterized by the accumulation of physiological, biochemical, clinical, and metabolic disorders that increase the risk of diseases related to obesity such as atherosclerotic cardiovascular disease and diabetes mellitus type 2, as well as mortality. The prevalence of MetS at a global level differs according to sex, age, race, and ethnicity of the population studied, and also on the basis of the definition of MetS that is used for diagnosis (4). There are various criteria for the diagnosis of MetS, with the National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III), and the International Diabetes Federation (IDF), which considers the ethnic and regional characteristics of patients, being among the most widely used sets (4,5). The adipose tissue participates actively in maintaining energy homeostasis through the secretion of adipokines. It is important to note that obesity often leads to deregulation and disrupting of the function of adipokines, which leads to various alterations including the metabolic syndrome (6). Adiponectin is one of the main adipokines secreted by adipocytes. The gene ADIPQ is located on chromosome 3q27 and is composed of three exons (7). Several studies have shown that adiponectin exerts the following functions that are beneficial to homeostasis: improves overall insulin sensitivity, enhances energy consumption and fatty acid oxidation, has anti-inflammatory and anti-oxidant properties, and contributes to cardiovascular protection (8). There is evidence of a connection between the 3q27 locus with type-2 diabetes, obesity, and metabolic syndrome (7). Several single nucleotide polymorphisms (SNPs) have been identified in ADIPOQ. Among these variations, the SNP -11377 C>G is located in the promoter region of the gene. The minor allele G alters the sequence of the binding site for SP1, which causes a loss of SP1 binding effect, probably affecting adiponectin promoter transcription activity (9). Studies in different populations have linked this SNP with metabolic alterations related to obesity (7,10). Mexico has a high prevalence of obesity and of risk factors associated with MetS. Thus, the objective of this study was to determine the allelic and genotype distribution of the ADIPQ gene polymorphism rs266729 (-11377 C>G) and its association with MetS in a Mexican population of western Mexico.
MATERIALS AND METHODS
STUDY POPULATION
We performed a descriptive, cross-sectional study. The population included 101 patients diagnosed with metabolic syndrome according to the ATP III criteria (11), who attended Centro de Salud Fresnillo 2 in Zacatecas, México, and 70 unrelated healthy subjects.
The study protocol was approved by the Institutional Ethics Committee (6/2017-2018). All participants provided their written informed consent, and the study was performed in accordance with the Helsinki Declaration (12).
ANTHROPOMETRIC PARAMETERS AND BLOOD COLLECTION
The values of height and weight for each participant were used to calculate their body mass index (BMI) (kg/m2). Also measured were the circumference of the waist and hip, and the waist-hip ratio. Finally, venous blood samples were collected from all study participants. The quantification of serum adiponectin levels will be performed in the next stage of the study.
GENOTYPING
Genomic DNA was extracted from peripheral blood leukocytes using a standard salting out method (13). The analysis of the ADIPQ -11377 C>G polymorphism was performed using a polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) method (10).
Briefly, PCR amplification (SureCycler 8800 thermal cycler) of a 334-bp fragment of the ADIPQ gene was carried out using a forward primer: GGTGGACTTGACTTTACTGG, and a reverse primer: TAGAAGCAGCCTGGAGAA. A 50 μL PCR reaction mixture containing 2 μL of genomic DNA, 10 μL of 10x Buffer Reaction (My Taq™ DNA polymerase, BIOLINE), 1.5 μL each of forward and reverse primers, 0.5 μL of My Taq DNA polymerase (My Taq™ DNA polymerase, BIOLINE), and 34.5 μL of water. The PCR conditions were as follows: an initial denaturation at 95 °C for 5 min, followed by 35 cycles, denaturation at 95 °C for 30 s, annealing at 60 °C for 30 sec, extension at 72 °C for 40 sec, and final extension at 72 °C for 4 min. Amplified fragments were analyzed through 6 % polyacrylamide gel electrophoresis stained with silver staining. The genotyping PCR products were digested with the restriction enzyme HhaI (New England Biolabs). The results of the genotyping were the following: CC genotype (334 bp), CG genotype (334, 212, and 122 bp) and GG genotype (212 and 122 bp).
STATISTICAL ANALYSIS
A descriptive analysis of the data (mean, standard deviation, frequency of genotypes and alleles, and contingency tables) and subsequently an inferential analysis (χ2 test, Student's t-test, and odds ratio with a confidence interval at 95 %) were carried out. These statistical analyses were performed using the software programs Prism 7 and IBM SPSS, version 25.0 for Mac OS X. Significance was assumed for p < 0.05.
RESULTS
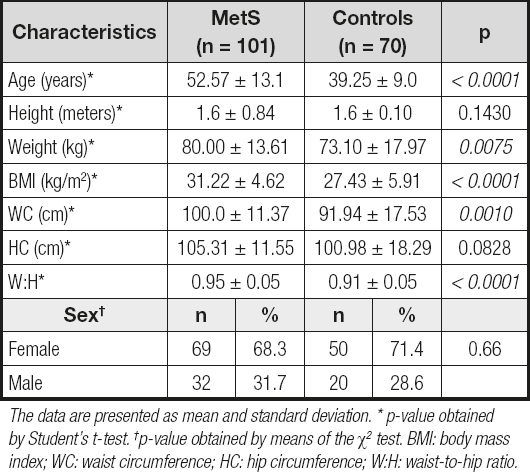
In the present study, a total of 171 people were enrolled, 101 MetS patients (mean age, 52.57 ± 13.1) and 70 unrelated healthy subjects (mean age, 39.25 ± 9.0). Table I shows their demographic characteristics.
Table I. Demographic characteristics of the study subjects
The data are presented as mean and standard deviation. * p-value obtained by Student's t-test. †p-value obtained by means of the χ2 test. BMI: body mass index; WC: waist circumference; HC: hip circumference; W:H: waist-to-hip ratio.
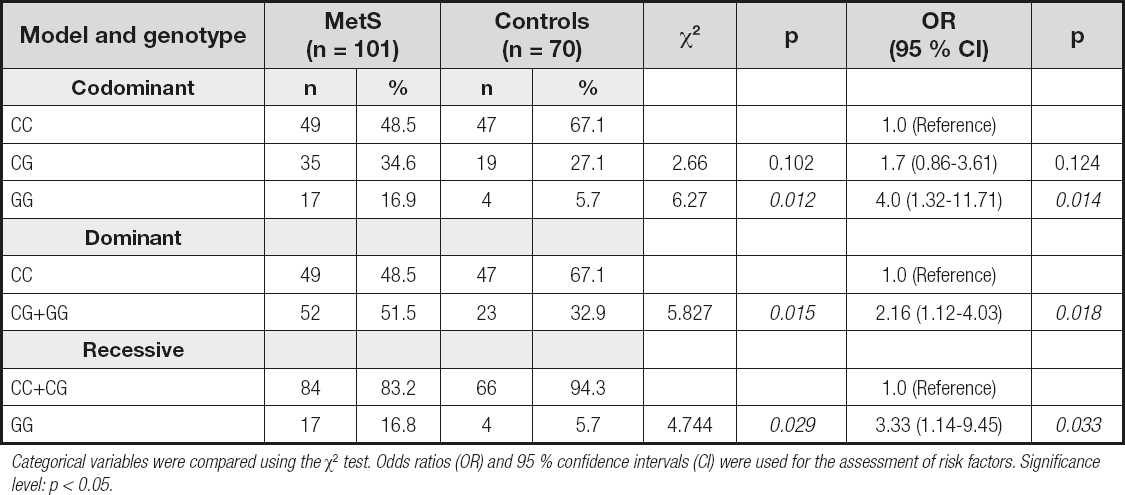
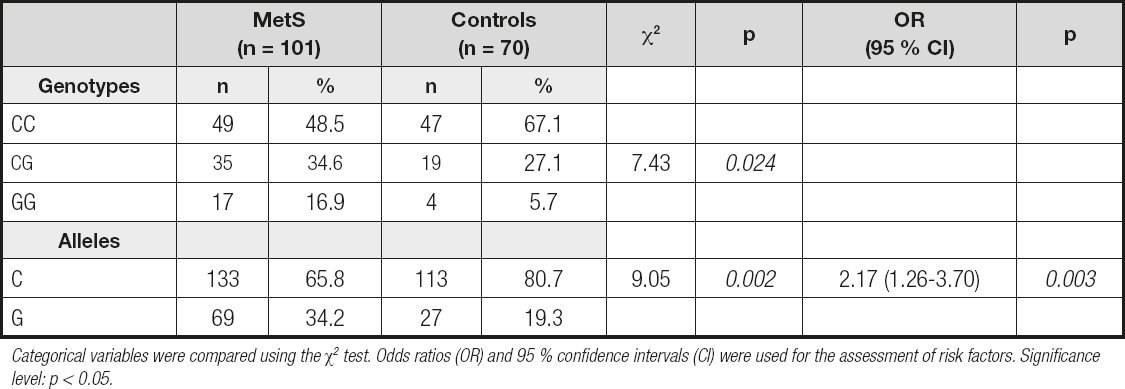
The distributions of ADIPQ -11377 C>G genotypes are shown in table II. The control group was found in Hardy-Weinberg equilibrium with a p = 0.283, whereas the group with MetS obtained a value of 0.021. The homozygous GG genotype was found to be more frequent in MetS patients (16.9 % for MetS vs 5.7 % for controls). The comparison between both groups showed that homozygous GG genotypes were significantly higher in patients than in controls (χ2 = 7.43, p = 0.024). Furthermore, we found a higher frequency of the allele G in MetS patients (34.2 %), as compared to what we observed in the control group (19.3 %) (OR = 2.17; 95 % CI: 1.26-3.70; p = 0.003).
Table II. Distributions of ADIPQ -11377 C>G genotypes and alleles in MetS patients and control subjects
Categorical variables were compared using the χ2 test. Odds ratios (OR) and 95 % confidence intervals (CI) were used for the assessment of risk factors. Significance level: p < 0.05.
The genetic model analysis showed that individuals carrying the GG genotype were at increased risk of MetS as compared to those with the CC genotype (OR = 4.0; 95 % CI: 1.32-11.71; p = 0.014). The CG + GG genotype (dominant model) showed a statistically significant correlation with MetS when compared to the CC genotype (OR = 2.16; 95 % CI: 1.12-4.03; p = 0.018), whereas the GG genotype (recessive model) was significantly associated with MetS risk when compared to the CC + CG genotype (OR = 3.33; 95 % CI: 1.14-9.45, p = 0.033) (Table III).
DISCUSSION
In the present study, we report an association between the ADIPQ -11377 C>G polymorphism and MetS risk in a Mexican population of western Mexico. Adiponectin plays an important role in insulin sensitivity, energy consumption, and fatty acid oxidation among other functions (8). Previous studies have shown that people with obesity and diabetes, as well as MetS, often have low levels of adiponectin in the serum or plasma (14-17). It is clear from current research that the levels of adipokines are influenced by body fat and inflammatory states, for example; in the particular case of adiponectin, in vitro and in vivo studies showed that tumor necrosis factor alpha (TNF-α) suppresses the multimerization and secretion of adiponectin (18). Likewise, obesity-induced adipocyte hypertrophy results in a shift in adipokine production from adiponectin to leptin (19). In fact, it has been proposed that adiponectin may have an important role in the pathogenesis of MetS (20). However, it is relevant to consider the genetic factors that could contribute to the variations seen in serum/plasma concentrations.
The ADIPQ -11377 C>G polymorphism is located in the promoter region of the ADIPQ gene (21). Reports in various populations of the world have shown an association of the ADIPQ -11377 C>G polymorphism with metabolic alterations related to obesity (7,10,22,23). In contrast, other studies have shown conflicting results with respect to the allele associated with type-2 diabetes (24), or even when the study population is classified by gender (9).
From another point of view, it would be important to evaluate whether the polymorphism has an impact on the modification of MetS components and other obesity-related diseases after a change in lifestyle such as a dieting. In this context, de Luis et al. showed that after weight loss, the CC genotype of the ADIPQ 11377 C>G polymorphism is associated with increases in adiponectin levels and decreases in low-density lipoprotein cholesterol, insulin, and homeostasis model assessment for insulin resistance after weight loss (25). Specifically, it was evident that after a monounsaturated fat-enriched diet, only subjects with the CC genotype showed significant improvements in total cholesterol (CC vs. CG + GG) (−9.0 ± 1.1 mU/L vs. −4.5 ± 2.4 mg/dL, p = 0.01), LDL cholesterol (−6.0 ± 1.1 mg/dL vs. −3.0 ± 0.9 mg/ dL, p = 0.03), glucose (−4.7 ± 1.1 mg/dL vs. −0.6 ± 0.5 mg/dL, p = 0.01), and insulin levels (−2.6 ± 1.0 mU/L vs. −0.7 ± 0.3 mU/L, p = 0.02), as well as in HOMA-IR (−0.5 ± 0.2 units vs. −0.2 ± 0.4 units, p = 0.03). The same improvement was reported after a polyunsaturated fat-enriched diet in all parameters. The trend continues with different diet treatments (26,27).
One interventional study in 60 extremely obese patients who were evaluated 32 months after bariatric surgery showed that SNPs in ADIPOQ gene were associated with changes in metabolic variables in obese individuals; for example, C-allele homozygotes presented a higher reduction in LDL cholesterol, total cholesterol, and triglycerides than carriers of the variant G-allele (28).
Few studies have focused on the relationship of this SNP with MetS. In the present study, the proportions found of the CC, CG, and GG genotypes in patients with MetS were the following: 48.5 %, 34.6 %, and 16.9 %, respectively, whereas in the control group the frequency of the GG genotype was only 5.6 %. Even though in an Italian population the genotype distribution of the group with MetS was comparable (CC, 38 %; CG, 33 %; and GG, 28 %) to that observed in our study, the control group showed a greater frequency for the GG genotype (40 %). On the other hand, a meta-analysis that evaluated the association between ADIPOQ polymorphisms and MetS in a Chinese population found no association with the ADIPOQ rs266729 polymorphism in any of the analyzed genetic models (allele model: OR = 0.98; 95 % CI: 0.82-1.17; dominant model: OR = 0.90; 95 % CI: 0.79-1.02; recessive model: OR = 1.09; 95 % CI: 0.85-1.39; homozygote model: OR = 1.03; 95 % CI: 0.80-1.33) (29). We found that the GG genotype was significantly associated with MetS risk under the codominant (OR = 4.0; 95 % CI: 1.32-11.71; p = 0.014), dominant (OR = 2.16; 95 % CI: 1.12-4.03; p = 0.018), and recessive (OR = 3.33; 95 % CI: 1.14-9.45; p = 0.033) genetic models. It should be noted that racial/ethnic diversity represents an important factor in the variability observed in the studies of SNPs, and other factors such as sample size, gender, or cut-off points for the classification of obesity can also provide variation in results.
Few studies have carried out a functional analysis of this polymorphism. Transcription factor Sp1 regulates the expression of a large number of genes, including adiponectin promoter activity (30). Zhang et al. found that the G-allele of the ADIPQ 11377 C>G polymorphism altered the sequence for one of the SP1 binding sites, which consequently resulted in the loss of the SP1 binding effect, and might play a role in the reduction of adiponectin promoter transcription activity (9). Further studies are required to elucidate the consequences of this SNP at the protein level and its interaction with other cellular components in the context of metabolic syndrome and diseases related to obesity.
Mexico is a country with a large amount of genetic diversity, and has a high prevalence of obesity, as well as of risk factors associated with MetS. An advantage of this study is that the selection of patients and controls was only performed in the western part of the country. A weakness of the study is the relatively small number of samples. Quantification of serum levels of adiponectin based on genotype would be useful to compare the effects of the ADIPQ 11377 C>G polymorphism in MetS patients.