INTRODUCTION
Fibromyalgia (FM) is a chronic rheumatic disorder whose etiology is not fully understood. It is characterized by generalized and widespread pain and several associated symptoms such as: stiffness, fatigue, non-restorative sleep, impaired cognition, depression, and reduced health-related quality of life (HRQoL). Women have a higher prevalence of this pathology. The prevalence of FM in studies carried out in the adult female population ranged between 2.4 % and 6.8 % (1). The ratio of females to males is 13.7:1 (2). It is also related to poor physical fitness and a high prevalence of overweight and obesity. Approximately 72 % of women with FM are overweight or obese (3). This high prevalence could be explained by the tendency towards physical inactivity FM patients exhibit (4). Sedentary habits, in turn, may be derived from and enhanced by the symptoms of FM, like pain, fatigue, or depression. FM is also closely related to high levels of cholesterol (5), high values of diastolic pressure (6), a higher waist-to-hip ratio (WHR), and a higher body mass index (BMI) (7), in addition to other common symptoms. A relationship between diabetes and FM was previously reported (8). In this regard, some FM-associated symptoms like memory impairment were suggested to be related to glucose metabolism abnormalities (7).
Current therapies are based on the treatment of symptoms through pharmacological and non-pharmacological therapies. Among pharmacological therapies, tricyclic antidepressants, cyclobenzaprine, tramadol, duloxetine, milnacipran, pregabalin, and gabapentin are recommended (9). Among non-pharmacological therapies, physical activity, psychological therapy, and sleep hygiene are widely accepted as helpful therapies (10). In the last few years, complementary and alternative nutritional treatments have been widely used. However, there is a need to establish the extent to which these nutritional treatments can improve FM symptoms.
Ganoderma lucidum (GL), also known as "reishi" or "lingzhi", is a type of mushroom widely used in traditional Chinese medicine. Some of the described effects included immunomodulation, anti-cancer, anti-diabetic, anti-inflammatory, anti-oxidant, anti-androgenic, anti-viral (including activity against HIV), anti-hepatitis, and cardio-protective effects (11). However, to our knowledge, there is no study focused on the assessment of the effects of GL in FM patients.
Ceratonia siliqua (CS) has been used in traditional Mediterranean medicine. CS flour is a high-fiber food (12), and a rich source of carbohydrates, proteins, and minerals. Among minerals, calcium, potassium, magnesium, sodium, and phosphorus are abundant (13). Effects of CS on blood cholesterol levels (14) and blood glucose levels (15) were previously reported. CS was suggested to reduce hypercholesterolemia and triglyceride levels. However, the reduction of cholesterol levels was only observed in the hypercholesterolemic group, whereas the normal-cholesterol group experienced an enhancement of their cholesterol levels (16).
The aim of the current paper is to evaluate the effects of GL and CS on blood glucose, lipid profile (cholesterol and triglycerides), WHR, blood pressure (systolic and diastolic), and anthropometric measures (weight, fat mass, and muscular mass).
MATERIALS AND METHODS
PARTICIPANTS
Participants were recruited from Spanish FM associations. Inclusion criteria were the following: a) being diagnosed with FM by a rheumatologist; b) being able to communicate effectively with the study staff; c) being aged more than 18 years; d) signing a written informed consent. Exclusion criteria included: a) being pregnant; b) changes in daily activity during the six weeks of treatment; c) being on immunosuppressants; d) having a diagnosis with diabetes; e) participation in other investigations; f) being on vitamin C supplementation; g) being on anticoagulants, and h) having been treated with GL and/or CS before. FM diagnosis was subsequently checked using an algometer (PainTest™ FPX 25 Algometer, Wagner Instruments, Greenwich, USA). All patients continued with their usual treatment during the study.
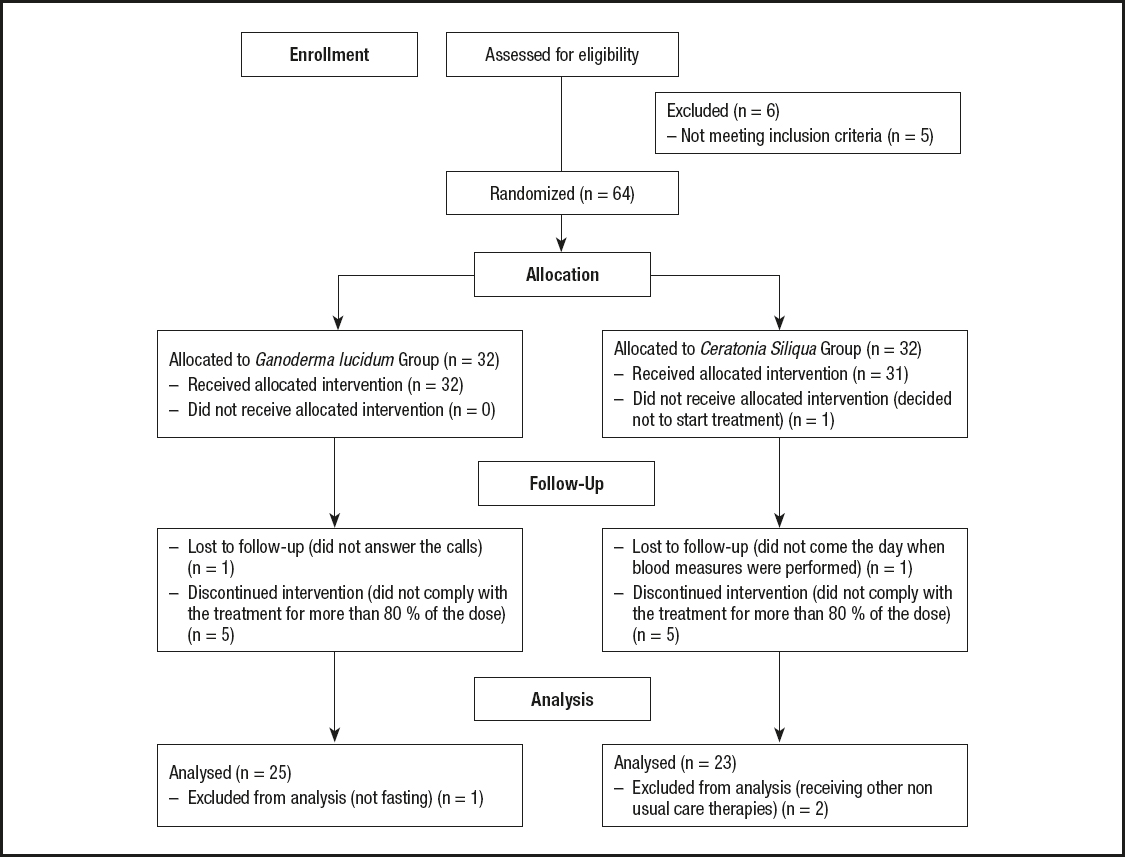
A diagram showing the flow of participants may be seen in figure 1. The initial sample comprised 70 FM patients. Five of them were excluded because they did not meet inclusion or exclusion criteria, and 1 participant voluntarily declined to participate. Therefore, a total of 64 women with FM were randomly allocated to the GL group (GLG) or CS group (CSG). All participants signed their written informed consent in accordance with the updated Declaration of Helsinki.
STUDY DESIGN
The current study was a randomized, double-blind pilot trial. Participants were randomly assigned to either the GLG (n = 32) or CSG (n = 32). Randomization was performed using a random numbered table, and assigning a code number to each participant. The double blind was kept until the end of the statistical analysis phase. The Bioethics Committee of Universidad de Extremadura (Spain) approved the current study, which was registered in the Australia-New Zealand Clinical Trials Registry (ANZCTR) with ID: ACTRN12614001201662.
PROCEDURE
Participants were allocated to one of the two groups: GLG or CSG. The doses used for both groups consisted of 6 g daily for 6 weeks, based on the review conducted (17-19). The GLG took 3 g of micro-milled GL carpophores dissolved in water twice a day, at breakfast and dinner. The CSG took 3 g of CS flour dissolved in water twice a day, too. Instructions were the same for the two groups. The GL and CS were provided by the company "MundoReishi Salud S.L.," located in the city of Palencia, Spain.
DATA COLLECTION
All outcome variables were assessed at baseline and after treatment. First, the determination of glucose, triglycerides, and total cholesterol levels by the Accutrend Plus System®: the Accutrend Plus device was used to measure glucose, triglycerides and cholesterol. The device determines lipidic parameters in capillary blood through a test strip that is inserted into the device. There are studies where they have evaluated its reproducibility, its validity, and its accuracy (20).
Blood pressure and heart rate were subsequently measured, and afterwards anthropometric values were evaluated using the Tanita body composition analyzer BC-418 MA and a tape measure. The Tanita is a device that estimates body composition through electrical bioimpedance. Anthropometric measures assessed included muscular and fat mass, weight, height, and waist and hip circumferences. The Tanita body composition analyzer BC-418 MA is a valid and reliable instrument (21). All anthropometric measures were assessed by the same researcher, at baseline and after treatment, in order to reduce variability among researchers (especially in waist and hip circumferences). One participant was not measured with this device because she had a pacemaker.
STATISTICAL ANALYSIS
All statistical analyses were performed using the IBM SPSS v.21 (Chicago, IL, USA) package. Values of the two groups were compared at baseline using Student´s t-test for independent samples. The distribution of data was determined by the Kolmogorov-Smirnov test. The results were expressed as mean and standard deviation.
The Pass v.11 software (NCSS, LLC. Kaysville, UT, USA) was used to calculate the statistical power of the fasting glucose variable. A repeated measures design with 1 between factor and 1 within factor has 2 groups with 32 subjects each for a total of 64 subjects. Each subject is measured 2 times. This design achieves 100 % power to test factor B if a Geisser-Greenhouse corrected F-test is used with a 74 % significance level, and the actual effect standard deviation is 25.10; achieves 100 % power to test factor W if a Geisser-Greenhouse corrected F-test is used with a 5 % significance level and the actual effect standard deviation is 33.45 (an effect size of 66.90); and achieves 100 % power to test the BW interaction if a Geisser-Greenhouse corrected F-test is used with a 5 % significance level and the actual effect standard deviation is 33.45.
Within-group changes after treatment were calculated using a paired t-test. The analysis of variance (ANOVA) for repeated measures was used in order to compare the effects of the two treatments. The level of significance was set at p < 0.05. To reduce the probability of making a type-I error, since multiple hypotheses are tested, the statistical significance of p was calculated with Bonferroni's correction, this being equal to 0.05/12 = 0.004. The effect size calculation was performed using Cohen's "d". According to Cohen's "d", effect sizes could be classified as small (from 0.2 to 0.49), medium (from 0.5 to 0.79), and large (> 0.8) (22).
RESULTS
No differences in main characteristics were seen between GLG and CSG at baseline (p > 0.05). Mean age was 55.92 (8.06) and 53.62 (11.74), respectively.
Figure 1 shows the flow of participants. Each group was initially made up of 32 women with FM. In the GLG, one participant was lost because she did not answer any call. Five participants were excluded because they did not take at least 80 % of the dose. Finally, 1 participant was excluded because she ate before fasting glucose measurement. On the other hand, 1 participant of the CSG decided not to start the treatment proposed, 5 women were excluded because they did not take at least 80 % of the treatment, 1 did not come the day when blood measures were performed, and 2 were excluded because they started receiving other non-usual care therapies. Therefore, a total of 48 women were analyzed in the efficacy analysis. Twenty-five of them belonging to the GLG and 23 to the CSG.
Tables I and II show the effects of GL and CS on the outcome measures. Two different analyses are reported: within-group and between-groups analysis. Results did not show any statistically significant differences for any of the outcome measures, both regarding the within-group and the between-groups analysis with Bonferroni's correction. However, without Bonferroni's correction, a significant reduction in fasting glucose levels was observed in the CSG when compared to baseline (Table I: within-group analysis). Glucose reduction was near 9 % (p = 0.045).
Table I. Effects of 6 weeks of GL and CS on women with FM: within-group analysis*
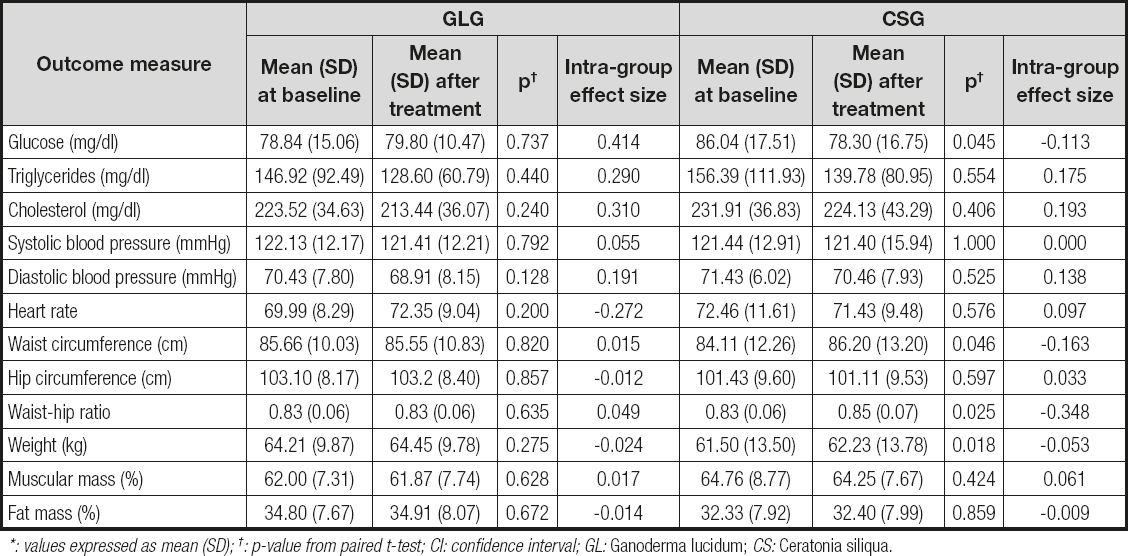
*: values expressed as mean (SD); †: p-value from paired t-test; CI: confidence interval; GL: Ganoderma lucidum; CS: Ceratonia siliqua.
Table II. Effects of 6 weeks of GL and CS treatment: between-group analysis*

*Values expressed as mean (SD); †: p-value from ANOVA for repeated measures; CI: confidence interval; GL: Ganoderma lucidum; CS: Ceratonia siliqua.
Without Bonferroni's correction we also found a statistically significant difference in the WHR of the GLG as compared to the CSG (Table II: between-groups analysis). This difference was due to an increase in waist circumference and then in WHR in the CSG. This group also gained weight significantly. In fact, the GLG experienced a reduction of less than 1 % in both WHR and waist circumference, whereas the CSG increased their WHR and waist circumference by around 2.5 %. No other anthropometrical changes were detected in both groups.
DISCUSSION
The current study reports a tendency of CS to reduce fasting glucose levels in women with FM. The effect of CS in reducing glucose levels is in accordance with other studies where a hypoglycemic effect of CS was reported (16,23,24).
Moreover, we found a WHR difference in the CSG as compared to the GLG. The WHR change was given because the CSG experienced a significant increase in waist circumference, whereas hip circumference remained unchanged in both groups.
An explanation for the between-group difference in WHR could be derived from the mean age of women participating in the study, 56.2 and 53.6 years for the GLG and CSG participants, respectively (data not shown), a range where most women are menopausal. It is known that physical activity in postmenopausal women tends to decrease as the cold season (fall and winter) approaches, as a consequence of environmental changes like: temperature, rain rate, number of daylight hours, and weather patterns (25). It is also know that reduction of physical activity (26) and weight gain (27) are related to an increase in WHR.
Considering that the administration of both GL and CS started in October and ended in December, the increase in waist circumference experienced by the CSG participants may be explained by both physical inactivity, because of the season the study was performed in, and CS intake. In fact, some studies in animals such as pigs (28) or rabbits (29) reported that a carob diet increased weight gain when compared to diets based on other foods. To test the influence of weight gain on WHR we made a regression analysis introducing as dependent variable the change in waist circumference, and as independent variable the change in weight. Results showed that only 15 % of the increase that occurs in the waist index could be explained by the weight gain in the carob tree group.
In contrast, GL intake could have mitigated the weight gain tendency trough an improvement of physical fitness (30), and the anti-obesity effects through both an inhibition of adipogenesis (31,32) and gut microbiota modulation (33).
Surprisingly, no significant differences were found in the within-group analysis of the GLG for glucose, and in both groups for cholesterol and triglycerides as reported in previous studies (34-37). This could be caused by the low doses of active compounds that were administered in our study, where 6 grams of the whole GL carpophore and CS fruit were used, in contrast to the quantity of active compound concentrate in the extracts used in other studies. For example, in the study by Hijikata and Yamada (1998), to obtain 1 gram of extract 17 grams of dried GL fruit were used. For this reason, considering that they are both authorized foods, and the safety shown in the current study, we suggest that doses of both GL and CS be increased in future studies with the whole GL carpophore or carob pod.
We decided not to use an extract because we wanted to be sure that all the potential beneficial spectrum of substances contained in the whole GL carpophore and CS fruit were used. In fact, extracts tend to concentrate only certain substances based on the specific method used for their extraction. Moreover, for this study we chose to use the minimum effective dose, based on the literature consulted as stated in the procedure paragraph, to avoid potential adverse effects that could result from an overdose, also considering the chemical hypersensitivity that can affect women with fibromyalgia (38).
In any case, our findings are important because they may benefit the 19.8 % of patients with FM who also suffer from diabetes (39). Moreover, the possible effects of GL on body weight should also be investigated as 72 % of FM female patients also have overweight or obesity (40).
To our knowledge, this is the first study that evaluates the effects of GL and CS on blood and anthropometric parameters in FM patients.
The current study has several limitations. First, absence of a placebo group. Second, seasonal changes were not controlled and could alter our results as discussed above. Third, the small sample size could hide some relevant findings. Fourth, there is a lack of references regarding the adequate dosing of both GL and CS. Fifth, a sample calculation was not performed for fasting glucose. We have calculated the statistical power of this variable. Finally, only non-diabetic women participated in the study. Thus, the reduction in glucose levels needs to be tested in patients with diabetes.