INTRODUCTION
Both developed and developing countries have modified their dietary patterns over the past few years. This pattern is associated with a higher intake of palatable foods, which are rich in saturated fats and refined carbohydrates, such as fast-food products, red meats, whole dairy products, and beverages with high content of simple sugars, characterizing a dietary pattern popularly known as the western or westernized diet (1).
In this dietary pattern lipids contribute about 40 % of the total energy ingested, especially in diets high in triglycerides, saturated fatty acids, and trans fatty acids (2), which influence metabolism and body composition (3). This food pattern is in line with Brazilian dietary habits and with the dietary patterns of other countries with western food patterns.
These habits are strongly associated in Brazil (4) with an increased prevalence of overweight (55 %) and obesity (19 %) and their correlated comorbidities, such as insulin resistance and glucose homeostasis imbalance (5), development of atherosclerosis (6), and a consequently increased risk of cardiovascular disease (7).
Based on dietary changes, recent evidence has shown the relationship between the pattern of meals taken throughout the day and the risk of overweight/obesity and associated diseases (8).
In western societies, lifestyle impacts show dinner as one of the meals with the highest energy intake, contributing ± 35 % of the daily calories ingested (9). Modern living has caused many people to switch their eating times from day to night in order to carry out their work activities. A change in the active period, exemplified by individuals on night shift work or other shifts, has been shown to be an additional factor of metabolic risk, in particular for obesity and cardiovascular disease (10).
Emerging studies have suggested that meal times or even diet composition can change the biological rhythm or circadian rhythm, thus influencing energy metabolism (11) as well as the regulation of digestive and absorptive processes (12). Situations of desynchronization of this rhythmicity, mimicked by changes in the quantity, quality, and timing of meals, are related to metabolic disorders and a consequent risk of increased body fat and its comorbidities (13).
In view of the aforementioned, we hypothesized that time-restricted feeding during 8 hours of the dark phase would worsen the biochemical profile of rats fed a westernized diet. Therefore, the aim of this study was to evaluate the effects of a westernized diet and of time-restricted feeding on the anthropometric and biochemical profile of adult Wistar rats.
MATERIALS AND METHODS
The experimental protocols were submitted and approved by the Ethics Committee on the Use of Animals (ECUA) of the Federal University of Pernambuco (UFPE) under protocol number 23076.017868/2016-36, and followed the standards of animal handling and care established by the National Council for Animal Experimentation Control, following the recommendations of the Brazilian Society of Science in Laboratory Animals (SBCAL) and the standards established in the Guide for Care and Use of Laboratory Animals (14).
ANIMALS AND EXPERIMENTAL GROUPS
The animals were kept in rooms with controlled temperature (22 ± 1 °C), relative humidity (± 55-60 %) and inverted cycle (8:00-20:00 h, dark cycle and 20:00-8:00 h, light cycle). Initially, forty male Wistar rats with 21 days of life were used. Half of the animals (n = 20) received a control diet and the other half (n = 20) received an experimental diet dubbed “westernized” (growth and maintenance phase) according to the nutritional needs recommended for the different life cycles of the animal (15). At 60 days of life the animals were arbitrarily divided into 4 groups, according to diet and temporal restriction of food. The restriction period imposed (8 hours of the active period of the animals was in the dark phase) was based on the study by Rocha et al. (10), which altered food availability to mimic the meal times of shift workers. Depending on the time of day, this restriction has been reported to alter energy homeostasis and cause metabolic disorders (16). The present study was designed to test metabolic outcomes when this restriction occurs during the active phase of the dark cycle. From the restriction protocol used new groups were formed: control (C, n = 9), restricted control (RC, n = 9), westernized (W, n = 10), and restricted westernized (RW, n = 8), totaling 36 animals kept in individual transparent cages during the subsequent 120 days. Four animals were excluded from the study due to the occurrence of esophageal perforation during the gavage procedure, and spontaneous respiratory disease.
DIETS, FOOD INTAKE, AND TIME-RESTRICTED FEEDING
The control group (C) received a standard commercial diet (Presence®: 26 % proteins, 63 % carbohydrates, 11 % lipids, and 3.6 calories/g) throughout the experiment. Group W received an experimental “westernized” growth diet (18 % proteins, 43 % carbohydrates, 39 % lipids, and 4.0 calories/g) until 60 days of life. After this age, the W groups received the same westernized diet but with proteins adjusted for the maintenance phase (14 % proteins, 46 % carbohydrates, 40 % lipids, and 4.1 calories/g). Just like humans, rodents need different amounts of energy and nutrients according to their life cycle (17). The diet was adapted from Ferro Cavalcante et al. (17), and its detailed composition is in table I.
Table I. Centesimal composition of the westernized experimental diet

Adapted from Ferro Cavalcante et al. (2013). g: grass; %: percentage; NaCl: sodium chloride; Na: sodium; BHT: butyhydroxytoluene.
After 60 days groups C and W were fed ad libitum throughout the 24-hour cycle, whereas the groups subjected to time-restricted feeding (RC and RW) received food after 8 hours of fasting during the dark cycle, e.g., between 8:00 h and 16:00 h. Food intake was measured by the difference between the amount of food offered and the amount rejected, in grams (g), as recorded on a digital electronic scale (brand Marte XL 500, class II; maximum capacity, 500 g; precision, 0.001 g). The accumulated amounts of food and energy intake were obtained weekly. The purpose of this control was to verify whether there was a contribution from the total amount food and energy intake on the outcomes observed under the assessed physiological and metabolic parameters.
ANTHROPOMETRIC MEASURES
Body weight was measured weekly until the end of the experimental period using the digital electronic scale (Marte® XL 500, class II; maximum capacity, 500 g, accuracy, 0.001 g). Body length (distance in cm from the nose to the base of the tail) was measured with the animal slightly anesthetized and relaxed. An inextensible measuring tape with a precision of 0.01 mm was used to measure length. Based on weight and length, the body mass index (BMI = weight/cm²) was calculated. At the end of the study, the abdominal circumference (measured at the anterior aspect of the hind legs) and thoracic circumference (measured at the posterior aspect of the forelegs), in cm, were obtained to support the assessment of anthropometric changes and risk of developing metabolic syndrome (18).
ORAL GLUCOSE TOLERANCE TEST AND CIRCADIAN RHYTHM OF GLUCOSE
An oral glucose tolerance test (OGTT) was performed between 123 and 146 days of life on the animals, in the light and dark photoperiods, in all groups. The animals were submitted to fasting for 6 hours and, after this period, the first blood sample (time 0) was collected for basal glucose measurement using the Accu-Check Performa®. Subsequently, a 50 % glucose solution was administered by gavage, at a dosage of 2 mg/g of body weight, for the oral glucose tolerance test (19); the glycemia was obtained at 30, 60, 90, and 120 minutes. The glucose area-under-the-curve (ΔG) was calculated using the trapezoidal method (20). The circadian rhythm of glucose was evaluated to observe whether there was an association with the temporal restriction of food and diet (21). On the same day, in all groups (± 120 days of life), every four hours, the rats were subjected to a capillary glucose test where blood was obtained through a small hole at the tip of the animal's tail.
BIOCHEMICAL PROFILE
After fasting for 10-12 h, the animals were euthanized using a muscle relaxant and an anesthetic (ketamine, 40 mg/kg and xylazine, 5 mg/kg), administered intraperitoneally, and then, through an intracardiac puncture, a blood sample (5 mL) was collected. Subsequently, the blood samples were centrifuged at 2500 g for 20 minutes, and the serum was collected and transferred to Eppendorf tubes, which were stored in a freezer at -20 °C for up to three months in order to be used for serological doses. Fasting serum glucose, total cholesterol and both high-density (HDL) and-low density (LDL) lipoprotein fractions, triglycerides, urea, and creatinine were measured. The analyses were performed using Biosystems reagent kits, as well as their methodology, through an automated instrument, the A15 Clinical Chemistry Analyzer (Biosystems®, Spain). The Friedewald equation (22) was used to estimate plasma LDL-cholesterol and VLDL-cholesterol levels through indirect calculation, using the formula: LDL-cholesterol mg/dL = Total cholesterol - HDL-cholesterol - (Triglycerides / 5).
STATISTICAL ANALYSIS
The data were analyzed using the Graphpad Prism 7.0 program (Graphpad Prism Software Inc.), and the normality of variables was tested using the Shapiro-Wilk test. All results are expressed as mean ± standard deviation error of the mean. For intragroup analyses before and after an intervention a paired Student's t-test was used. For the analysis of body weight and food intake, with diet and time as cofactors, a two-way analysis of variance (ANOVA) for repeated measures (RM) was used, followed by Bonferronís post-hoc test when a difference between groups was observed. For analyses with only one variable a one-way analysis of variance (ANOVA) was used, followed by Tukey´s post-hoc test when a difference between groups was detected. Significance was considered for p < 0.05.
RESULTS
SOMATIC GROWTH AND BODY COMPOSITION
At 60 days of life, the weight of the animals in the W group was 11 % higher than that of animals in C (C = 245.0 ± 16.1 g; RC = 247.3 ± 26.0 g; W = 272.4 ± 16.9 g; RW = 290.3 ± 9.4 g, p < 0.005). The final weight (± 180 days of life) in the RC group was around 20 % lower than in its unrestricted pair (C = 404.6 ± 39.1 g; RC = 335.1 ± 36.5 g, p < 0.001), and around 10 % lower in RW as compared to the group fed the unrestricted westernized diet (W = 488.9 ± 51.2 g; RW = 438.8 ± 36.5 g, p < 0.001). As regards restricted groups, RW was about 30 % heavier than RC (RC = 335.1 ± 36.5 g; RW = 438.8 ± 36.5 g, p < 0.001), and the difference between C and W was 21 % (C = 404.6 ± 39.1 g; W = 488.9 ± 51.2 g, p < 0.001) at the end of the experiment.
The food restriction imposed while still in the growth phase of the animals impaired longitudinal growth regardless of dietary treatment (C = 25.99 ± 0.91 cm; RC = 24.48 ± 0.94 cm; W = 26.00 ± 1.07 cm; RW = 24.32 ± 1.11 cm, p = 0.001). When analyzing body mass index, the results showed a higher BMI in RW when compared to RC (C = 0.649 ± 0.053 g/cm2; RC = 0.577 ± 0.031 g/cm2; W = 0.737 ± 0.107 g/cm2; RW = 0.801 ± 0.067 g/cm2), in line with the greater body mass weight in the RW group.
Body perimeters showed a greater thoracic circumference in groups fed the westernized diet, whether restricted or not, when compared to their unrestricted and restricted control pairs (C = 14.67 ± 0.58 cm; RC = 13.84 ± 0.44 cm; W = 15.88 ± 0.78 cm; RW = 16.64 ± 1.02 cm, p < 0.001). The abdominal circumference showed no differences between restricted and unrestricted groups on the same dietary treatment, but was strongly responsive to the westernized diet (C = 15.94 ± 0.80 cm; RC = 16.38 ± 0.98 cm; W = 18.66 ± 1.21 cm; RW = 19.29 ± 1.21 cm, p < 0.001). Also, differences in relative gonadal fat (C = 1.45 ± 0.28 g %; RC = 1.41 ± 0.23 g %; W = 2.45 ± 0.97 g %; RW = 1.95 ± 0.77 g %, p < 0.05) and retroperitoneal fat (C = 1.76 ± 0.84 g %; RC = 1.83 ± 0.61 g %; W = 3.19 ± 1.00 g %; RW = 3.91 ± 1.15 g %, p < 0.05) were observed.
DIETARY INTAKE
The accumulated dietary intake (Fig. 1A) over the weeks of experimentation revealed a difference in food intake between the restricted control group (RC) and its unrestricted pair (C), as well as between RW and RC, and between W and C. However, the same was not observed for the restricted westernized group (RW) and its pair (W) (C = 1,500 ± 111.4 g; RC = 1,189 ± 132.3 g; W = 1,049 ± 151.2 g; RW = 995.4 ± 98.63 g, p < 0.001).
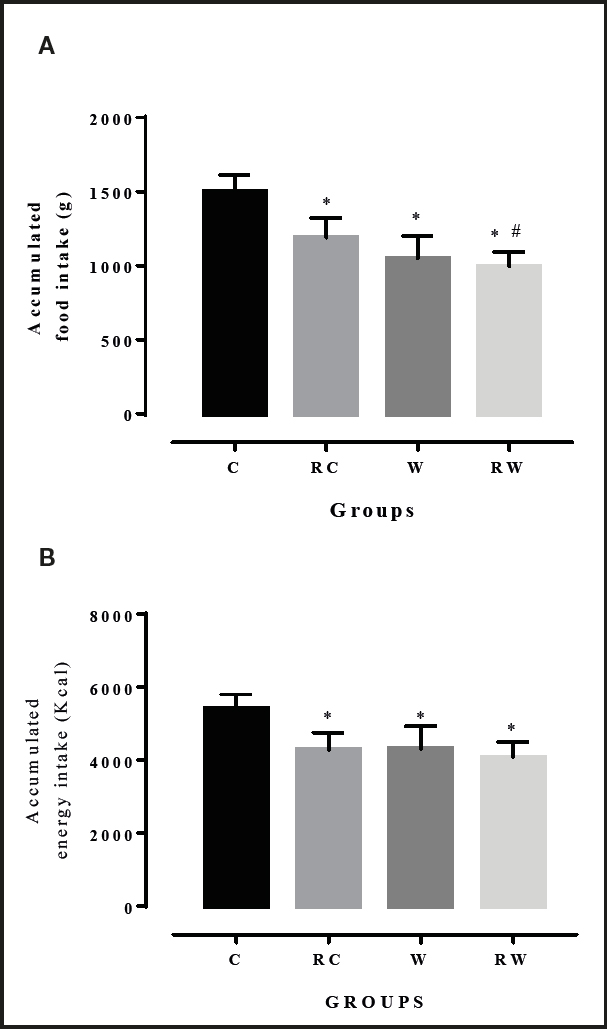
Figure 1. Dietary intake (A) and energy intake (B) accumulated over 8 weeks of experimentation. Groups (n, 8-10 per group) — C: control; RC: control with time-restricted feeding; W: westernized; RW: westernized with time-restricted feeding. Two-way ANOVA repeated measurements using Bonferroni's post-hoc test. *: vs. C; #: vs. W (p < 0.05)/Graphpad Prism 7.0.
The energy value ingested (Fig. 1B) showed that, despite the fact that the westernized diet was more caloric by 13 %, the results were similar except between the RW and RC groups, which did not differ from each other (C = 5,399 ± 401.2 kcal; RC = 4,279.0 ± 476.2 kcal; W = 4,302 ± 619.8 kcal; RW = 4,081.0 ± 404.4 kcal, p < 0.001).
The data set suggests that the metabolic and physiological alterations observed in the present study are not only due to differences in energy but also to differences in the proportion of macronutrients in the diet.
CIRCADIAN RHYTHM OF GLUCOSE
The follow-up of the 24-hour glycemic curve (Fig. 2) for the groups with ad libitum diet (C and W) (Fig. 2A) showed that group W presented a higher glycemia as compared to C in the three intervals of the light phase (20-24 h; 24-04 h, and 04-08 h). In the restricted groups, it was observed that the RW group showed lower glycemia levels than W in the last two intervals of the dark phase (12-16 h and 16-20 h). Although a reduction in blood glucose in the RW group was observed when compared to group W, glycemia remained higher than in its restricted counterpart (RC) at various points in the interval, revealing that the circadian rhythm of glucose is sensitive to both the temporal restriction of food and the nutritional composition of the diet.

Figure 2. Circadian rhythm over glucose in a 24-hour cycle, between groups throughout the cycle (A) and intragroup in light phase and dark phase (B, C, D, E). (A) C: control; RC: restricted control; W: westernized; RW: restricted westernized. Two-way ANOVA for repeated measures followed by Bonferronís post-hoc test. *: vs C; δ: vs RC; #: vs W (p < 0.05). (B) CL: light control; CD: dark control. (C) RCL: restricted light control; RCD: restricted dark control. (D) WL: light westernized; WD: dark westernized. (E) RWL: restricted light westernized; RWD: restricted dark westernized. ∆: dark period; □: light period. Two-way ANOVA for repeated measurements followed by Tukey´s post-hoc test/Graphpad Prism 7.0.
In the evaluation of the intragroup circadian rhythm of glucose as a function of the light and dark phases of the 24-hour cycle, for the control (Fig. 2B) and westernized (Fig. 2D) groups fed ad libitum no difference was observed. In the RC group (Fig. 2C) the difference between the light and dark phase occurred at two points on the curve, at 12:00 hrs and 16:00 hrs, consistent with the dark phase during which the animal was without food. Interestingly, the RW group shows no differences at any point on the curve (Fig. 2E), suggesting that the composition of the diet may interfere with the mechanisms of glycemic control in a different manner than in the group fed a control diet.
ORAL GLUCOSE TOLERANCE TEST (OGTT)
The OGTT obtained in the dark phase of the 24-hour cycle (Fig. 3A) showed that the RW group presented higher blood glucose levels differing from the RC group. In the light phase of the cycle (Fig. 3B) group W differed from C at 90 and 120 min, and RW differed from W at 90 min. The results are corroborated by the incremental glucose area-under-the-curve of the above-mentioned groups (Fig. 3A and 3B), and the differences between the results of the OGTT performed in either the light or dark phase suggest that these values may be associated with the endogenous rhythm of insulin secretion or sensitivity of insulin-dependent tissues.
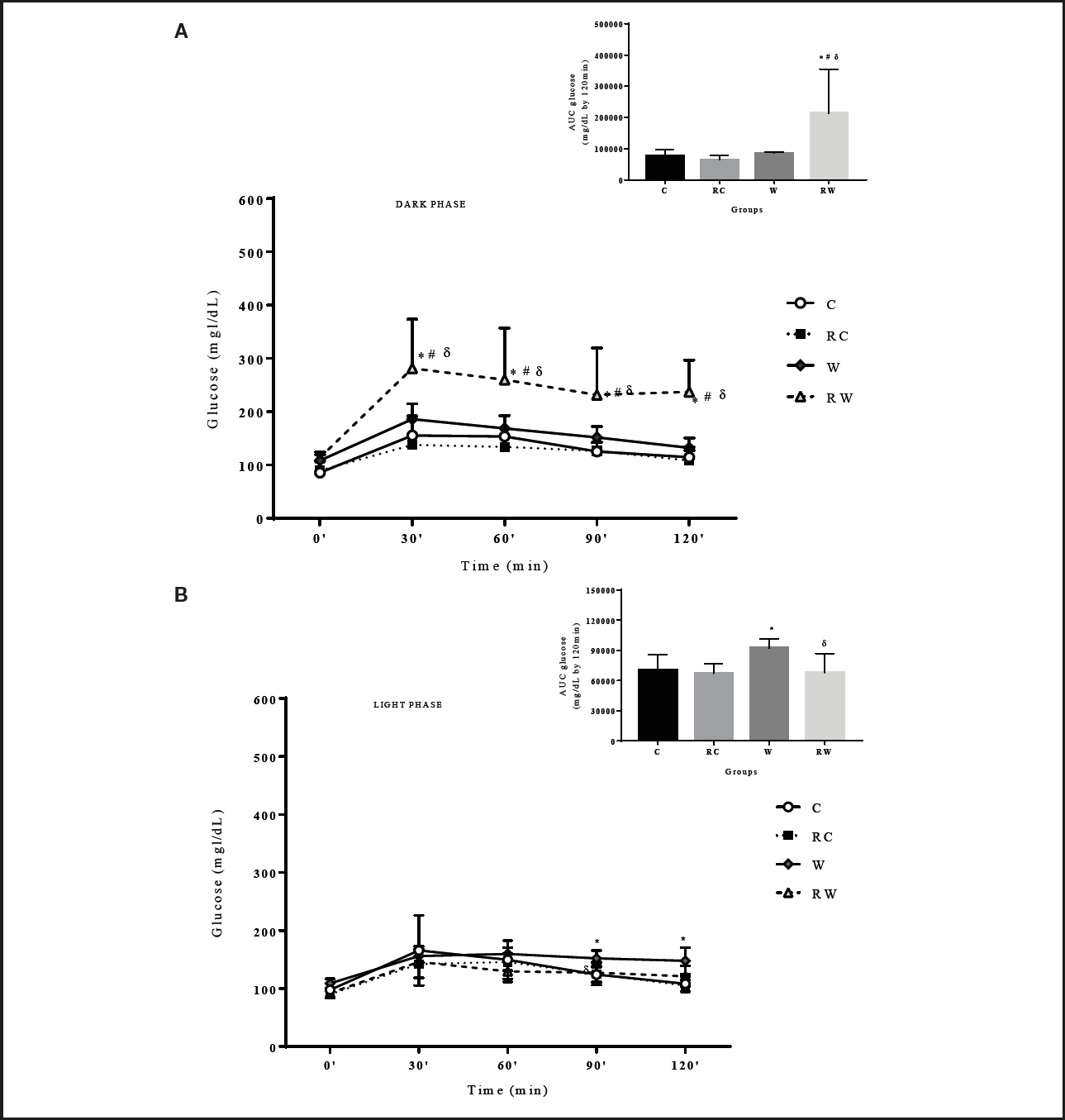
Figure 3. Oral glucose tolerance test (OGTT) and glucose area-under-the-curve according to the dark (A) and light (B) phases among groups (n = 8-10 per group). C: control; RC: restricted control; W: westernized; RW: restricted westernized. Repeated measurements two-way ANOVA for OGTT and one-way ANOVA for glucose area-under-the-curve followed by Tukey´s post-hoc test/Graphpad Prism 7.0. *: vs C; #: vs RC; δ: vs W (p < 0.05).
BIOCHEMICAL PROFILE
The biochemical analysis showed a reduction in serum urea levels in RC, W, and RW, and increased creatinine levels in RC and W as compared to C. On the other hand, we highlight that the experimental interventions were more deleterious for lipid rates than for blood glucose. Fasting glucose levels (C: 189.5 ± 29.9 mg/dL; RC: 205.3 ± 34.4 mg/dL; W: 180.3 ± 24.4 mg/dL; RW: 202.0 ± 18.4 mg/dL, p > 0.05) did not show any differences between groups, but mixed dyslipidemia was observed in groups W and RC when compared to group C. The results found for the TG/HDLc ratio, however, were not significant, probably because of an increase in HDLc. Interestingly, the results of time-restricted feeding in the group fed the control diet proved to be more deleterious for lipid changes than those recorded in the group fed the westernized diet (Table II) when RW was compared to the C group and unrestricted W group.
Table II. Biochemical parameters of the adult rats submitted to westernized diet and/or time-restricted feeding

C: control group; RC: time-restricted feeding in control group; W: westernized; RW: time-restricted feeding in westernized group. One-way ANOVA followed by Tukey´s post-hoc test. *: vs. C; †: vs. RC; ǂ: vs. W (p < 0.05). Values expressed as mean (MD) ± standard deviation (SD).
DISCUSSION
The original experimental design of the present study is the first to investigate the relationship between westernized diet and dietary restriction, and their impacts on anthropometric measures and glycemic and lipid profiles. Concerning the repercussions on the body weight of the study animals, the westernized diet and the time-restricted feeding produced these effects: the westernized diet was effective in increasing body weight; and temporal restriction of food caused a reduction in body weight. Changes in the biochemical profile and differences in oral glucose tolerance, however, according to the phases of the circadian cycle, indicated a dependence on the diet as well as on the food restriction and its interactions.
Previously (23), it was observed that the induction of obesity by consumption of a high-fat diet is more effective when started early and continued for several weeks; this may result in a weight increase of 10-20 % when rodents reach adulthood. Similarly, the present study also found a weight increase starting at 60 days of life. Both groups, restricted and unrestricted, fed a westernized diet were heavier than their respective pairs fed a control diet.
In this study, the higher body weight found in the westernized group was not associated to the amount of food or energy that was taken in, but rather associated with higher metabolic efficiency, which is characterized by a reduction in the energy expended by metabolically active tissues, with an increase in energy conservation (24). Schilperoort et al. (25) verified that a temporal restriction of food during the dark period, alternating with weeks of food restriction during the light period, with no reduction in the total amount of food ingested, caused a long-term reduction in basal energy expenditure, a reduction in lean mass, and an increase in fat mass. The effect of westernized diet on body composition was also verified by a high amount of abdominal fat (gonadal and retroperitoneal), as well as by greater abdominal and thoracic circumferences. This result is consistent with that observed in 2012 by Angéloco et al. (26), who found an increase in abdominal and thoracic circumferences by 17 % and 20 %, respectively, in animals fed a westernized diet containing 50 % of lipids.
The lower food intake seen in the westernized groups may in part be associated with high satiety as caused by the presence of lipids in the gastrointestinal tract. A previous study observed a relationship between fat molecules in the gastrointestinal tract and slow gastric emptying (27,28), as well as an increase in the secretion of hormones such as cholecystokinin, glucagon-like peptide 1 (GLP-1), and peptide YY (PYY) in intestinal mucosal cells, with suppression of hunger and reduced food intake (29).
On the other hand, an increase in the metabolization of fatty acids, as a function of the excess fat available or of increased mobilization, raises the concentrations of malonil-CoA, and can alter metabolism in hypothalamic neurons by modulating the expression of peptides that regulate food intake (30). For instance, a reduction in the levels of transcripts encoding orexigenic neuropeptides, such as neuropeptide Y and agouti-related peptide (NPY/AgRP) (31,32), while increasing transcript encoding for anorexigenic neuropeptides, such as pro-opiomelanocortin and cocaine- and amphetamine-regulated transcript (POMC/CART) (32,33), can lead to suppressed hunger and smaller amounts of food ingested.
The composition of the diet as well as the temporal restriction of food also influenced the circadian rhythm of glucose. The follow-up of glucose rhythm between the light and dark photoperiods of the 24-hour cycle in each group demonstrates that the composition and photoperiod of the diet exerts an influence on the circadian curve of glycemia. Besides food intake as modulator of insulin secretion, this hormone has a cyclic oscillation. The insulin secretion rhythm has been previously demonstrated over 24 hours (34). The sensitivity of insulin-dependent tissue can be modulated both by the suprachiasmatic nucleus (central clock of the circadian timing system) and by oscillators or clock genes located in peripheral organs (35). Thus, the biological rhythms of blood glucose and glucose tolerance present a circadian pattern where the highest concentration and highest glucose tolerance are found at the beginning of the active period, and gradually decrease towards the end of the day or light day (34). Given this, the differences seen within each group, relative to the light or dark phase, are not only derived from food restriction but also from cyclical sensitivity to insulin (5), causing glucose intolerance (19) and incremental glucose area-under-the-curve levels.
Environmental cues such as diet can modify the expression of key enzymes in glucose metabolism (36), as well as affect the genes that maintain glycemic homeostasis in the fasting state (37). Rodents fed a high-fat diet have reduced glucose tolerance, but when subjected to time-restricted feeding show improvement in glucose tolerance and a reduction in insulin resistance (16), in contrast to what was found in the present study. The restricted westernized group (RW) did not improve its glycemic response in the oral glucose tolerance test, especially during the dark pase of the cycle. However, the phase of the circadian cycle when blood glucose is monitored seems to affect the results. Intriguingly, although animals fed westernized diets presented glucose intolerance, fasting hyperglycemia was not observed. This fact suggests the development of hyperinsulinemia, which characterizes the condition of mild glucose intolerance, which in turn appears before any detectable elevation of plasma glucose concentration (5).
Mixed hyperlipidemia (high triglycerides and cholesterol) was found in animals fed a westernized diet. It is possible that hypertriglyceridemia may be related, at least in part, to the absence of any interruption of the lipogenic and lipolytic mechanisms in the liver and adipose tissue, respectively, as a consequence of hyperinsulinemia (38). In the liver, the synthesis of fatty acids from glucose and amino acids, called new lipogenesis, results in the packaging of triglycerides in very low-density lipoproteins (VLDL) that are exported in the blood and captured by peripheral tissues (39). This lipogenic route, in the presence of high levels of fat and simple sugar in the diet, as occurs in the westernized diet pattern, may have its usual stimulation amplified, leading to overproduction of lipids (5).
In adipose tissue, marked lipolysis can occur because of a low retention of triglycerides in the unilocular droplets of fat due to a decrease in the expression of the adipocytic proteins of lipid droplets such as perilipin, which are responsible for the storage and retention of lipids in this tissue (40). These and other metabolic pathways may be concomitant and are responsible for various alterations found in our research as a result of the westernized diet offered to the animals.
The body's biological response to time-restricted feeding was diet-dependent. The time-restricted feeding performed in the RW group improved the regulation of lipid levels. However, this result was not observed in the RC group, which presented higher values of total cholesterol, HDL, LDL, VLDL, and triglycerides when compared to the unrestricted control group. In contrast, another study has shown that animals exposed to food restriction for 12 hrs during four weeks underwent a reduction in serum cholesterol levels by 29 % and 36 % according to the light or dark phase of the 24-hour cycle, respectively, even with no reduction in body weight (41). On the other hand, the animals submitted to time-restricted feeding for 16 weeks showed a lower body weight and reduced levels of triglycerides, total cholesterol, and LDL, similar to what was found in the present study (42).
In summary, this study describes the destructive repercussions of westernized diet on glycemic homeostasis, anthropometric parameters, and biochemistry profile. Curiously, time-restricted feeding caused more adverse metabolic effects on animals fed the control diet than in animals fed the westernized diet. We also demonstrated that the circadian rhythm of glucose and the oral glucose tolerance test vary according to the phase of the circadian cycle, and are related to diet and food restriction. Moreover, food restriction in animals fed a westernized diet ameliorated the lipidic parameters, but this “benefit” needs to be evaluated, together with other metabolic factors such as other periods and the number of hours daily when the restriction occurs, as well as the investigation of intracellular events in key organs for metabolism such as liver tissue, adipose tissue, and skeletal muscle tissue.














