INTRODUCTION
Over the last decade, our diet and lifestyle have changed in relation to economic development. In terms of health, nutritional status, eating patterns, and physical activity, this evolution has been negative. As a consequence, there has been a significant increase in chronic noncommunicable diseases and obesity, the prevalence of which has increased and continues to increase at alarming levels in our society (1). According to Keaver et al., overweight and obesity will reach levels of 89 % and 85 % in males and females, respectively, by 2030 (2). In Spain, the most recent data on the prevalence of obesity correspond to the ENPE study, which found an average prevalence of 21.6 % among adults aged 25 to 64 years (22.8 % in men and 20.5 % in women) (3). Obesity substantially increases the risk of diabetes, cardiovascular disease from increased blood pressure and altered blood lipid profile (4,5), certain types of cancer (colorectal, breast, endometrial, renal, esophageal, and pancreatic) (6), and other diseases. Also, as body mass index (BMI) increases (especially when it is ≥ 30 kg/m2) the relative risk of mortality gradually increases. Obesity has been associated with poorer quality of life, a greater frequency of disability and health services use, and higher economic costs (7).
Breast cancer is the most frequent malignant neoplasm in women worldwide, and is the type of tumor that causes the highest number of deaths in Spain. It accounts for 18.2 % of cancer deaths in women and is the leading cause of death in women between 40 and 55 years. Its incidence is increasing, especially in developed countries, in which 50 % of all cases of breast cancer occur (8). Although the incidence has increased, the mortality rate has remained stable in recent years, a benefit attributed to early detection programs and advances in systemic treatment. One in every 10 women will develop breast cancer at some point in her life. Standardized average survival according to age in Europe is 93 % at 1 year and 73 % at 5 years (9). In Spain, breast cancer has a 5-year survival rate of more than 90 % (10).
Obesity and breast cancer are related: the treatment breast cancer entails is associated with a greater probability of weight gain and the development of overweight and obesity, and the presence of obesity has been associated with poorer breast cancer outcomes. The etiology of weight gain associated with the treatment of breast cancer may be partly explained by a decrease in physical activity resulting from increased feelings of fatigue. This reduction can reach up to 50 % of the usual activity of these women (11). In a study conducted by Lynch et al., patients with breast cancer spent most of the day in sedentary or low-intensity activities (12). However, physical activity has been associated with a protective effect on the development of breast cancer and its recurrence due to its influence on hormone levels, insulin resistance, and hyperinsulinemia (13-15).
Although there are not sufficient data to confirm the role of estrogen metabolites as predictors of breast cancer, a reduction in the circulating levels of primary estrogens may lower the risk of breast cancer in postmenopausal women. During menopause, however, these estrogens are reduced, leading to increased estrogen metabolism from adipose tissue and skin; thus, increased estrogen is directly related to an increase in fatty tissue (16). In addition, various studies have found an association between weight gain and a poorer evolution of breast cancer or its recurrence. This situation could be explained by the inflammatory and hormonal changes that obesity produces, which could favor the growth of tumor-related hormones such as estrogens, androgens, insulin, leptin, etc., as well as the oxidative stress linked to this disease (17,18). Pan et al., in a recent review, found that women with breast cancer and obesity who lost weight after their diagnosis had a reduced risk of recurrence and mortality compared with those who maintained a normal weight, since obesity might be associated with altered hormonal profiles that stimulate tumor development (19). Recent studies have observed that women with overweight or obesity at the time of diagnosis had a 50 % increase in their probabilities of developing a second, more aggressive tumor when compared with those with normal weight (20-22). Finally, women with obesity 1 year before breast cancer diagnosis could have an increased risk of weight gain and mortality as compared with those with normal body weight (23).
Few studies have evaluated the importance of developing multidisciplinary follow-up programs including the prevention and management of obesity (23). Kwiatkowski et al. proposed using nutritional intervention, nutrition education, physiotherapy, psychological support, and physical activity programs for women with breast cancer in the post-chemotherapy phase for 1 year to achieve weight loss. At the end of their study, a significant reduction in weight, as well as in waist circumference, were observed (23). Moreover, significant reductions in body weight had been observed in women who had survived breast cancer after undergoing a nutritional and physical activity intervention, in comparison with a control group (24). In a recent study conducted with overweight and obese women with breast cancer, Cheryl L. Rock et al. concluded that a nutritional intervention resulting in weight loss can significantly reduce cancer recurrence.
However, once the literature is reviewed, we hypothesize that not only a nutritional intervention, but also the effects of nutritional education along with physical activity (the intervention group [IG]), compared with following a nutritional intervention program alone, without nutritional counseling or physical activity (the control group [CG]), in women with breast cancer after a 6-month supervised intervention period and at 1 year of follow-up, might result in an improvement in body weight and in lipid profile, and could provide benefits in terms of breast cancer recurrence and survival.
MATERIALS AND METHODS
STUDY PARTICIPANTS
For the present study, the Medical Oncology and Gynecology Department recruited the participants, who had been evaluated at the Clinical Nutrition Department, La Paz University Hospital (HULP), Madrid, Spain. The criteria to be met to be eligible for the study were as follows: age older than 18 years; female sex; newly diagnosed breast cancer; having a suitable understanding of the clinical trial; agreeing to voluntarily participate in the study; and signing the informed consent. Exclusion criteria were as follows: patients with disseminated disease; receiving drug treatment for weight loss; suffering from an eating disorder; with mental illness; with low cognitive ability; or with problems complying with the general dietary and physical activity recommendations. Withdrawal criteria from the study included: death or lack of attendance to more than 2 treatment sessions.
All participants gave their informed consent to take part in the study, which was approved by the Scientific Research and Ethics Committee of HULP (Code 3114) in accordance with the ethical standards of the Declaration of Helsinki (25).
STUDY DESIGN
The study took the form of a randomized, prospective, controlled clinical trial lasting 24 weeks, and included 1 year of follow-up. The participants (n = 65) were randomly assigned to 1 of 2 treatment groups: CG or IG. Patients in the CG received a nutritional intervention consisting of an individualized diet; patients in the IG received the same nutritional intervention and also nutritional education and individual physical activity sessions.
NUTRITIONAL INTERVENTION
All participants received an individualized diet according to baseline energy expenditure, as measured with a bioelectrical impedance analyzer (BIA), and the corresponding recommendations for physical activity according to the World Health Organization (WHO) (26). Dietitians at the Clinical Nutrition Unit, HULP, used a food exchange list as a tool for dietary modification, and prepared menu samples in accordance with the healthy eating recommendations issued by the Spanish Society of Community Nutrition (27). This tool helps to develop customized meals, facilitating adherence to a diet. Family and work aspects, as well as mealtimes, were also taken into account.
In cases where weight reduction was required, the main goal was to promote a reduction in energy intake relative to expenditure, aiming for a 200-400 kcal/day deficit. Participants were reminded of the menu monthly, at each visit, and reinforced monthly at each visit throughout the 24 weeks of intervention. A participant's menu was assessed at the beginning, during, and at the end of the intervention period with a 72-hour dietary recall and a validated food intake frequency questionnaire to assess dietary compliance.
NUTRITIONAL EDUCATION
Once a month, a dietitian at the Clinical Nutrition Department, HULP, presented nutritional education sessions, 5 in total, of 1 hour each. The topics covered were as follows:
Generalities on obesity: definitions and diagnosis of obesity; complications and treatment of obesity.
Principles of nutrition: energy, macronutrients, and micronutrients; food groups, recommended distribution, and nutritional pyramid.
Situations of daily life: how to shop for food and cook healthily; how to eat outside the home.
Special situations: food myths and beliefs, miracle diets; learning to manage anxious moments and avoid temptations.
Physical activity: the importance of physical activity in breast cancer.
PHYSICAL ACTIVITY
The women in the IG attended 4 sessions per week: once a week, they performed a supervised session by the Rehabilitation and Physiotherapy Service, HULP, for 24 sessions in total, to perform aerobic and anaerobic exercises. Physical activity information for each attendant was collected through the International Physical Activity Questionnaire (IPAQ) (28) at the beginning of the intervention, at the end, and after 36 weeks. The supervised workouts were divided into two groups:
- Aerobic training: cardiovascular work with big muscle groups. These exercises were performed with a cycle ergometer at < 75 % of the maximum heart rate determined by an initial test. During the exercises, they made progressive increments with a goal of 45 minutes during 4 days, followed by cooling and stretching movements.
- Anaerobic training: after two weeks of aerobic training, they started to strengthen smaller muscle groups (biceps, triceps, deltoids, etc.) with weights according to a 40-60 % of their maximum repetition technique. They made three sets of ten repetitions for each muscle groups twice a week.
In addition, they also performed three physical activity sessions per week at home: study participants recorded information on their home training sessions including date, duration, heart rate before, during, and after each session, effort assessment according to the Borg scale, and incidents, and reported it to the Rehabilitation and Physiotherapy Service at HULP.
ANTHROPOMETRIC VARIABLES
Anthropometric measurements were taken at the beginning and at the end of each intervention period using standard techniques, and adhering to the international guidelines set out by the WHO (29). All measurements were made by trained personnel in the morning, with the participant barefoot and wearing only underwear. Body composition was determined using a BIA, the ElectroFluidGraph analyzer (Akern s.r.l., Florence, Italy). Height and waist circumference were measured and recorded adhering to the international norms set out by the WHO. BMI was calculated using the following formula: weight (kg) / height (m)2.
HEALTH VARIABLES
Information was collected on medical conditions and medications taken. Blood pressure and heart rate were measured on the right arm using a Spot Vital Signs 420 automatic monitor (Welch Allyn, Madrid, Spain) (accuracy: ± 5 mm Hg). Three measurements were taken at 5-min intervals, and the means were calculated. The populations were classified according to whether the participants exhibited prehypertension or high blood pressure that had remained undiagnosed and was not being treated pharmacologically.
BIOCHEMICAL VARIABLES
Blood samples for general biochemistry testing were collected early in the morning at the HULP Extractions Department at the beginning of the intervention, at the end of the 24 weeks of treatment, and after 36 weeks. Samples were kept at 4 °C to 6 °C until they were analyzed, which was always performed within 48 h. Biochemical serum lipid profile (e.g., total cholesterol [TC], high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], triglycerides), glucose, and protein levels were measured by an enzymatic spectrophotometric assay using an Olympus AU 5400 apparatus (Izasa; CA, USA).
STATISTICAL ANALYSIS
Data were analyzed using repeated measures (group x time) for within-group and between-group changes. Primary outcomes were body weight; body composition parameters; BMI; waist circumference; bicipital, tricipital, subscapular, and suprailiac folds; and heart rate. Secondary endpoints were physical function (includes aerobic and anaerobic sessions of physical activity) collected through the IPAQ questionnaire; food frequency; and lipid profile serum biomarkers: glucose, TC, HDL-C, LDL-C triglycerides and proteins. The statistical power was determined through a calculation based on the precision of the estimate of the standard deviation and by the treatment effect size.
Compliance and adherence were measured during the study using a specific questionnaire evaluated in the Clinical Nutrition Department, and a subject was considered in the analyses when she verified more than 70 % of compliance verification (nutritional intervention).
The Physiotherapy Service at HULP verified the activity carried out through questionnaires to verify compliance with and adherence to physical activity. Once the adherence was verified, investigators of the Clinical Nutrition Department carried out the statistical analysis of the IPAQ questionnaire to evaluate the physical activity of the subjects.
Changes from baseline to week 48 (1 year) were defined by the difference in the value of a parameter at week 48 minus the value at baseline. Sensitivity analyses were conducted around the assumption of missing data. A p value of < 0.05 was determined to indicate significance. Descriptive statistics were calculated (the mean and standard deviation for quantitative variables, and percentages for qualitative variables). All analyses were performed using SPSS v.26.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
RECRUITMENT AND STUDY POPULATION
The study was performed between February and June 2013. A total of 65 women (50 ± 9.43 years old) with breast cancer were eligible for inclusion. Some 18 participants were lost at 6 months (12 in the CG and 6 in the IG) due to personal reasons (n = 15) and failure to follow treatment instructions (n = 2). Thus, 47 participants completed the 24-week study. During the 1-year follow-up, 12 women were lost to personal causes (n = 6 in each group). Therefore, 35 women ultimately completed the follow-up, and only their results were included in the subsequent analyses (Fig. 1). The baseline characteristics of the 35 women who completed the study were found to be comparable between the two groups. The protocol compliance (nutrition intervention and physical activity) was high and no differences were observed between groups. Also, no significant baseline differences in baseline diet were noted between the groups.
HEART RATE, BODY WEIGHT, AND BODY COMPOSITION OF PARTICIPANTS BY TREATMENT, AND GROUP
Table I shows the results of heart rate, body weight, and body composition. Blood pressure and heart rate remained within normal values for the general population (120/80 mm Hg) and did not differ between groups neither at baseline, nor after the intervention or after 1 year of follow-up (p > 0.05) (Table I). In contrast, there were significant changes in body weight and BMI in the IG at the end of the study (p > 0.05) (Table I). There were no significant differences between groups in waist circumference or in tricipital, bicipital, suprascapular, or suprailiac folds, nor any changes in any of the parameters from baseline to any time point, which allowed to obtain accurate values in the variation of body composition (% of fat-free mass, % of lean mass, and % of fat mass) (p > 0.05) (Table I).
Table I. Change in measurements per participant (X ± SD)

Data are expressed as mean ± SD. BMI: body mass index; FFM: fat-free mass; LM: lean mass; FM: fat mass; M0: month 0; M6: month 6; M12: month 12; a: differences after the intervention period between the groups. Significant differences between the start and the end of the intervention periods (*p < 0.05).
BIOCHEMICAL VARIABLES
The effects of the interventions on blood lipids, glucose, and protein levels between groups are shown in table II. Despite randomization, there was an imbalance between groups at baseline in TC and protein concentrations, with higher baseline values in the IG. This imbalance was corrected, however, and a significant reduction in LDL-C in the IG occurred between the intervention period and baseline (M6-M0) (-7.33 ± 41.96 vs. 12.80 ± 25.32). There was also a significant difference in TC levels between groups in the same period (2.58 ± 50.55 vs. 9.31 ± 34.37). Although there was no significant reduction in TC at the end of the study (M12), the TC reduction between groups was relevant (-29.29 ± 39 in the IG vs. 6.08 ± 51.87 in the CG) (Table II). In addition, there was a significant difference in triglyceride levels between groups between the 6-month intervention period and the 1-year follow-up (M12-M6) in favor of the IG. On the other hand, the interventions did not significantly affect glucose, HDL-C, or protein values in any group.
Table II. Blood lipids, glucose, and protein levels at baseline, postintervention, and at 1 year of follow-up
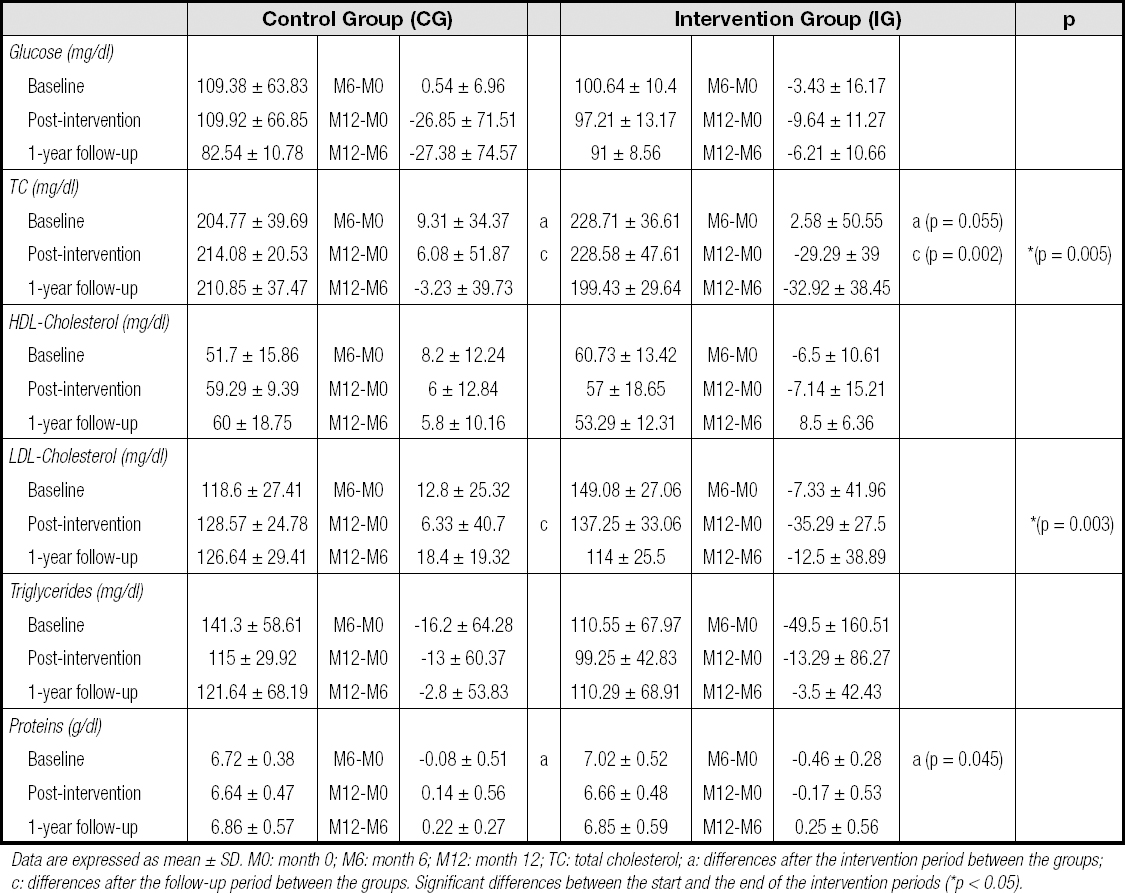
Data are expressed as mean ± SD. M0: month 0; M6: month 6; M12: month 12; TC: total cholesterol; a: differences after the intervention period between the groups; c: differences after the follow-up period between the groups. Significant differences between the start and the end of the intervention periods (*p < 0.05).
DIETARY INTAKE
The mean dietary intake values are outlined in table III. Although no significant differences were observed between the CG and IG at the end of the intervention period, both groups increased their daily intake of grains, fruits, oily fish, dairy products, and oils.
Table III. Results of the food frequency questionnaire during the study period

Data are expressed as mean ± SD. There were no significant differences between the start and the end of the intervention periods or in the change recorded between the intervention periods.
Both groups had reduced their consumption of red meat and sweets at the end of the intervention period; however, no significant differences were observed between them (Table III). These data were useful for examining group differences, although this approach will not permit an exact characterization of the dietary intake of any given individual due to day-to-day variations in intake.
PHYSICAL ACTIVITY
The main results of the physical activity questionnaire are shown in table IV. No significant differences were detected in any type of physical activity variable between the CG and IG at the end of the intervention (M6-M0), between the follow-up period and the intervention period (M12-M6), or at the 1-year follow-up (M12-M0).
Table IV. Results of the physical activity questionnaire during the intervention and follow-up

Data are expressed as mean ± SD. M0: month 0; M6: month 6; M12: month 12; a: differences after the intervention period between the groups; c: differences after the follow-up period between the groups; b: differences after the follow-up period.
However, it was observed that the IG was less sedentary at the end of the 1-year follow-up, spending fewer hours a day sitting, but without statistical significance. Similarly, both groups showed increased physical activity at the 1-year follow-up visit, with no differences between groups (Table IV).
DISCUSSION
The aim of this study was to determine the effect of a 24-week nutrition education program, including nutritional intervention, nutrition education, and physical activity sessions in women with breast cancer. The nutritional intervention comprised a balanced diet adjusted to the energy requirements of each volunteer in both groups. In addition, the IG participated in 5 nutritional education sessions. According to public health policies, the prevention and/or management of chronic diseases involves following a healthy diet, maintaining adequate levels of body mass, reducing sedentary lifestyles, and increasing physical activity (30).
The women in our IG lost, on average, 1.87 ± 3.41 kg of body weight after the 1-year follow-up period. The difference in body weight and consequently in BMI was significant between groups at the end of the study (p > 0.05) (Table I). This reduced body weight in the IG group was similar to that resulting from other studies with a similar design, which could be related to the effectiveness of the nutrition education program. Also, physical activity might help to control body weight in patients with breast cancer (31). Lastly, the slight and nonsignificant decrease in heart rate observed at the end of the intervention period could be a consequence of weight loss and the effect of training (Table I).
Some body composition parameters (% of fat free, lean, and fat mass) revealed no differences between groups at the end of the study (Table I). Recent studies have shown that the method of assessing body composition is not precise enough to measure small changes in body fat over time (32). Nutritional education for women with breast cancer had a beneficial effect on their dietary pattern, which in turn could have had a beneficial effect on body weight and BMI.
In this study, both groups made consistent changes in their diet. Based on the food frequency questionnaire, they increased their consumption of grains, fruits, blue fish, dairy, and oils, and reduced their consumption of red meat and sweets. These results could be associated with a better quality of life. In a recent study, Hebert et al. had concluded that a nutrition education program improved the dietary pattern of women with breast cancer, and that they continued to maintain this pattern after the intervention period, resulting in a significant reduction in body weight (32). Similarly, Anderson et al. had found that physical activity and a 6-month nutrition education program in women with breast cancer produced a significant loss of body weight, a significant reduction in saturated fat consumption (-4.2 % vs. -1.2 %; p = 0.013), and a significant increase in fruit and fiber consumption (0.5 servings vs. 0.0 servings; p = 0.006; 4.8g per 1000kcal vs. 1.3g per 1000 kcal; p = 0.007, respectively) (33) compared with a control group (34). Therefore, a dietary intervention and a physical activity program could improve the dietary pattern of women with breast cancer, achieving a healthier eating profile and leading to reduced body weight and better quality of life.
Physical activity is well known to improve strength and daily activity in clinical populations. Our IG reported a reduction in the number of sitting hours compared with the CG (-3.55 ± 3.75 vs. -0.85 ± 4.98). Although no significant differences were observed, the IG group slightly decreased their physical activity level after the intervention period during six months (total IPAQ: -132.45 ± 1232.22), and then they increased it after a year (total IPAQ: 234.33 ± 1177.41). These results are in accordance with other studies, and may be due to complications arising from cancer treatments. Subsequently, patients gained sufficient skills, confidence, and knowledge to focus on long-term physical activity, and to achieve a greater adherence to exercise as participants felt that physical activity represented a way to feel better. In contrast, the women who were in the CG did not receive physical activity recommendations and consequently did not improve their physical activity after the one-year follow-up period. These results are in accordance with recent studies, such as that by Carayol et al. These researchers found that a comprehensive physical activity therapy with aerobic and endurance exercises, as well as dietary counseling, compared with a control group, reduced levels of fat mass and BMI, and increased muscle endurance in women with breast cancer (35).
Our results, showing significant reductions in TC and LDL-C in the IG, are in agreement with the literature investigating the prevention of cardiovascular disease. The literature suggests that increased physical activity and nutritional education can influence lipid profiles in patients with cancer, increasing their survival rates (36). Our results are in line with a recent study that demonstrated the effect of physical activity in patients with breast cancer using recommendations for exercises that could be performed at home, compared with a control group without recommendations. At the end of the intervention period, both BMI (-6 kg/m2; p = 0.02), TC (-38 mmol/L; p = 0.001), and LDL-C (-3 mmol/L; p = 0.023) were significantly lower as compared with the control group (37). We might not have found reductions in glucose, HDL-C, and protein concentrations due to an insufficient sample size to detect differences between groups. Lahart et al. had concluded in a recent study that patients with invasive breast cancer who performed physical activity with recommendations for exercises that could be performed at home could reduce their TC and LDL-C concentrations when compared with a control group. Similarly, another study conducted by Swisher et al. had shown that the combination of aerobic exercise therapy and dietary counseling reduced BMI (2.4 % vs. 0.4 %, p < 0.05), suggesting that exercise and healthy eating can be effective in breast cancer survivors as compared with a control group (38).
The strengths of this study involve a randomized design, a long follow-up period for 1 year in total for each analyzed patient, a wide range of outcomes including both subjective and objective measurements, and an intention-to-treat approach. Limitations include the final sample size after one year of follow-up. This factor should be taken into account for future studies. It will be necessary to take losses into account, which may be due to the consequences of breast cancer treatment (tiredness, treatment, discomfort, medications, and other diseases). Moreover, self-reported measurements such as the food frequency questionnaire and IPAQ require participants to recall past activity, and represent a subjective means of estimating individual physical activity and dietary pattern levels. Further studies are warranted and should be required to establish physical activity programs and dietary advice as part of any institutional action protocols for women with breast cancer.
CONCLUSIONS
We found that a nutrition education program (including nutritional intervention, nutritional education, and physical activity) resulted in significant favorable effects on body weight, BMI, TC, and LDL-C in women with breast cancer compared with a control group, who received only a nutritional intervention. Reductions in body weight were correlated with improvements in lipid profile. Therefore, dietary interventions and physical activity in patients with breast cancer provide positive effects and could be important therapy components to reduce breast cancer recurrence and increase breast cancer survival. Further studies should examine the long term effects of a lifestyle program in patients with breast cancer.















