Dear Editor,
The clinical investigations reported in this journal employ the standard framework of frequentist statistics based on significance assumptions (p < 0.05). This method leads to a dichotomization of results as “significant” or “nonsignificant”, which has been questionable in the face of unstable replicable findings (1). The use of the Bayesian approach allows for an improved way of drawing statistical conclusions from clinical data since it facilitates answering the question, “what is the probability that the effect is conclusive based on the data?” to provide greater validity to significant conclusions.
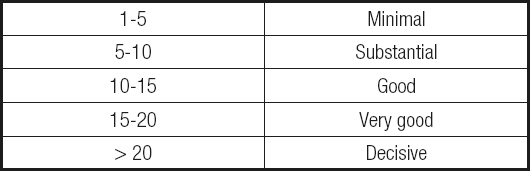
For example, the most common method is the Bayes factor (BF), which estimates the probability of one hypothesis relative to another based on the study sample (null vs. alternate hypothesis) (2). This model allows to quantify the weight of confirmatory evidence (WoE) in favor of the significant effect hypothesis by estimating the decimal logarithm of the BF multiplied by 10 (3,4), where values greater than 20 report a decisive weight of evidence (Table I). Such statistical parameters favor a more intuitive interpretation of the results for clinicians, who need to make clinical decisions according to the evidence of reproducible clinical findings.
It is possible to evaluate the WoE of significant findings with various statistical values (e.g., d, f, OR, Z-test, AUCROC) (5,6) because of their conversion to effect size (ES) using an online calculator (7), favorable for including various convertible ESs in future quantitative systematic investigations.
Two studies in the present journal were considered - the first included 365 hospitalized patients and evaluated the diagnostic prediction of overall mortality by the CONUT method of detecting malnutrition risk level with an AUCROC value of 0.644 (8). The other meta-analytic investigation (7 articles and 817 participants) confirmed the hypothesis that slow eating is a protective factor against excessive food and energy intake with a significant estimate of Z = 4.46 (9). The respective statistical transformations were performed: the former reported a value of r = 0.252 and the latter estimated a coefficient of r = 0.156, respectively.
For the FB results, the sample data and the convertible effects of the AUCROC (FB = 9200), and of the meta-analytic effect (FB = 980) were considered; such estimates indicate an extreme evidential strength (the results are supported by the data beyond variation and random error) (1) with a weight of compelling evidence of 91.19 for the first study and 68.88 for the second, respectively.
The inclusion of WoE values allows to quantify the practical credibility of statistical conclusions in clinical research beyond the questioning of the single use of significance hypotheses, which allows reinforcing future research in the present journal. This is inclusive of other Bayesian models applicable in health sciences such as the binomial test or the Bayesian A/B test, used in the current context of COVID-19 (10).