INTRODUCTION
Hyperlipidemia indicates abnormally elevated levels of lipids or lipoproteins due to abnormal fat metabolism or function, and the diagnostic standard of hyperlipidemia has been illustrated in the related guidelines (1,2). Hyperlipidemia can have a direct impact on the structure and function of vessels and the heart, leading to various cardiovascular complications (3,4). Obesity refers to excessive total and/or local fat content and abnormal distribution caused by genetic and environmental factors, and a link has been found with hyperlipidemia by lipid biomarkers according to previous research (5-7), which might provide a new idea to treat serum lipid levels in these obese individuals. There is likewise some people with other diseases that affect serum lipid levels, such as diabetes, hypothyroidism, Cushing's disease, and so on. For these kinds of patients, their primary disease may be treated to maintain serum lipid levels at a normal range (8). However, for non-obese healthy people with hyperlipidemia, which means that none of the situations above is applicable, the cause of their rising levels of serum lipids and how to control their serum lipids appropriately needs further exploration. At present, statins have a high effect in lowering LDL-C and are widely used in the clinical setting. Although statins are generally well tolerated, they are linked to numerous adverse effects like gastrointestinal events, respiratory infections, headaches, and muscle-related symptoms, including bilateral muscle pain, weakness, and inflammation. A long-term application will cause liver dysfunction, etc. It is also found that there is an increased risk of glioma with statin use according to a recent study (9). For those with hyperlipidemia who are unable to tolerate statins and those who have potential indications for non-pharmacological treatment of hyperlipidemia, it is critical to find a safe and effective way to lower serum lipid levels.
In recent years, people are more and more interested in using nutraceuticals to manage serum lipid levels. As one of the nutraceuticals, probiotics are a type of living microorganism that comes from the host and promotes the health of the host with safety and rarely side effects. Probiotics can adjust the microbial community in the intestinal tract, regulating the immune system and improving the anti-oxidative system by producing microbial components and metabolites (10-12). With the advantage of probiotics, there are numerous clinical trials (13-28) assessing the use of probiotics for the treatment of hyperlipidemia. Some systematic reviews (29-33) have supported their hyperlipidemia role built on randomized controlled trials, though their results have some limitations. For example, the studies they included in their reviews are often evaluating other primary diseases, making their results less rigorous. One of the systematic reviews (33) showed the role of probiotics in obese or overweight patients with hyperlipidemia, and the rest did not consider the factor of obesity, so there are no special reviews showing the effect of probiotics in non-obese patients, which means a further evaluation needs to be carried out. A large number of studies can potentially be missed if literature searches are restricted to English-only sources; interventions are not serious in some of the trials included before, such as the use of soy bean, which could also lower serum lipid levels by bioactive peptides probably according to previous research (10,34,35). As a consequence, it will influence the facticity of the results. Besides, the intervention measures or subjects are relatively limited.
Therefore, a systematic review and meta-analysis was conducted to evaluate the effect of probiotics on non-obese healthy adults with hyperlipidemia.
MATERIALS AND METHODS
PROTOCOL
This meta-analysis followed the Preferred Reporting Items for Systematic Meta-Analysis (PRISMA) statement (36). The protocol was registered at PROSPERO (registration number: CRD42020176302).
SEARCH STRATEGY
Several electronic databases were searched for available research studies by the authors: PubMed, Embase, the Cochrane Central Register of Controlled Trials, and Web of Science. All of the databases above were systematically searched from their commencement to January 2021 for relevant literature. A manual search of references of the included articles and reviews was performed for additional omitted studies. The specific search strategies were as follows:
((((“Hyperlipidemias" [Mesh]) OR ((((((((((((((((((Hypercholesterolemia[Title/Abstract]) OR Hyperlipemia[Title/Abstract]) OR Hyperlipemias[Title/Abstract]) OR Hyperlipidemia[Title/Abstract]) OR Lipidemia[Title/Abstract]) OR Lipidemias[Title/Abstract]) OR Lipemia[Title/Abstract]) OR Lipemias[Title/Abstract]) OR Cholesterol[Title/Abstract]) OR Triglycerides[Title/Abstract]) OR TGs[Title/Abstract]) OR HDL-cholesterol[Title/Abstract]) OR HDL-C[Title/Abstract]) OR LDL-cholesterol[Title/Abstract]) OR LDL-C[Title/Abstract]) OR lipid profile[Title/Abstract]) OR plasma lipids[Title/Abstract]) OR serum lipids[Title/Abstract]))) AND ((“Probiotics" [Mesh]) OR (((((((((((probiotic[Title/Abstract]) OR culturelle[Title/Abstract]) OR lactobacillus[Title/Abstract]) OR bifidobacterium[Title/Abstract]) OR enterococcus[Title/Abstract]) OR streptococcus[Title/Abstract]) OR clostridium butyricum[Title/Abstract]) OR bacillus[Title/Abstract]) OR yogurt[Title/Abstract]) OR yoghurt[Title/Abstract]) OR fermented milk[Title/Abstract]))) AND (((randomized controlled trial[Publication Type]) OR randomized[Title/Abstract]) OR placebo[Title/Abstract]).
SELECTION CRITERIA
The references retrieved were evaluated by two independent investigators (Sun and Liu) by scanning the title and abstract according to the inclusion criteria, and then screening again by reading the full text. If there were any disagreements, they would be resolved by consensus and asking the third party (Wang) to resolve the disagreements, or by contacting the original author of the article if necessary. The inclusion criteria and the exclusion criteria are shown in table I.
DATA EXTRACTION
The following data were collected and organized from the eligible studies by two independent investigators (Sun and Liu): the name of the first author; publication year; country; study design; characteristics of enrolled subjects (number, age, gender, race and BMI); interventions including strain, dose, form of probiotics, and duration; and baseline TC levels.
QUALITY ASSESSMENT
The quality of the included studies and the risk of bias were assessed by two independent investigators (Sun and Liu) according to the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0, which contains seven criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other bias. These seven criteria were rated as “low risk" , “unclear risk" , or “high risk" depending on the characteristics of each criterion reported in the study.
STATISTICAL METHODS
The effect of the probiotics on serum lipid levels was measured by the weighted mean difference (WMD) between the intervention and the control groups at follow-up. Before the meta-analysis was performed, the lipid levels in mg/dL and mg/L were all converted into mmol/L, and standard errors or confidence intervals (CIs) were converted to standard deviation (SD) for the analyses. The changes in serum TC, LDL-C, HDL-C and TG of subjects in each group in the selected references were extracted and expressed in the form of mean ± SD (x ± s). As several included studies did not offer the net changes in SD of serum lipids from baseline values, we calculated the SD change by the following formula (1):
SD change = √ [(SD pre-treatment) 2 + (SD post-treatment) 2 - (2* coefficient *SD pre-treatment *SD post-treatment)]
The coefficient was taken as 0.5 according to other eligible studies that provided the SD at baseline, final SD, and SD change.
This meta-analysis was performed using the STATA 15.1 software (Stata Corp., College Station, TX). The heterogeneity of the studies was evaluated by Cochran's Q statistic and I2 test. Studies were considered homogeneous if the p-value of the Q-test was > 0.1 or the I2 value was < 50 %; else they would be considered heterogeneous. We chose a random effects model to analyze data.
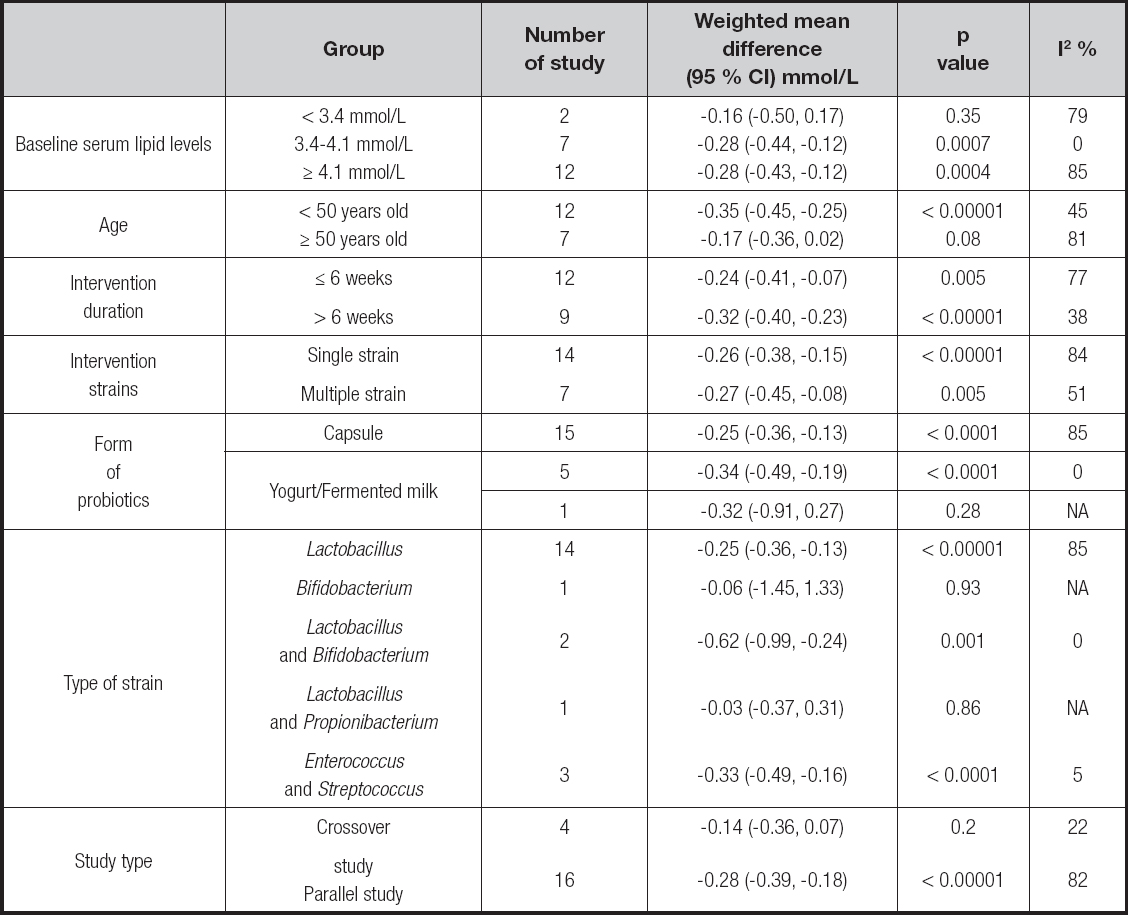
To further explore the other factors which could influence the results, a series of subgroup analyses was performed by Review Manager 5.3 (Cochrane Collaboration, 2014), including baseline serum lipid levels, age, intervention duration, strain, form of probiotics, type of strains, and study type, with p < 0.05 considered statistically significant. The sensitivity analysis was done by using the leave-one-out method to examine the impact of each study on the results.
Publication bias was evaluated using funnel plots and statistically assessed by Egger's regression test as performed by the STATA 15.1 software (Stata Corp., College Station, TX). If the funnel was asymmetric, or the Egger's test p-value was < 0.05, publication bias would be considered to exist.
RESULTS
DESCRIPTION OF STUDIES
A total of 2103 studies were retrieved on the basis of the search terms and search strategies described above. There were 1285 studies to be screened by titles and abstracts since 818 studies were removed because of repetition. Of the 1285 studies, 51 studies remained to be read full-text for further exclusion. Finally, 16 studies (13-28) were selected with the inclusion criteria (Fig. 1). Of the 16 studies, 5 studies (13,17,18,20,23) which applied more than one intervention duration were regarded as multiple independent trials. Thus, a total of 21 independent trials with 1429 participants were included in this meta-analysis. All the participants included in the trials met the diagnostic conditions of hyperlipidemia. The characteristics of each study are shown in table II.
Table II. The characteristics of each study

RCT: randomized controlled trial; DB: double blind; SB: single blind; P: parallel design; C: crossover design; NM: not mentioned; E.: Enterococcus; S.: Streptococcus; L.: Lactobacillus; B.: Bifidobacterium; P.: Propionibacterium; CFU: colony forming units.
QUALITY ASSESSMENT
The Cochrane Risk of Bias tool was used to assess the methodological quality of the included studies, which are presented in figure 2 and figure 3.

Figure 2. Risk-of-bias graph showing the authors' judgements about each risk-of-bias item presented as percentages across all the included studies.

Figure 3. Risk of bias summary showing the authors' judgements about each risk-of-bias item for each included study.
Most of the 16 studies were of low risk and high quality. In the generation of random sequence, 4 studies (14,23,26,27) did not describe specific methods, while 6 studies (13,15,17,21,23,28) did not specifically describe allocation concealment; with regards to the blinding method, there were 13 double-blind studies [13,14,16-26] involving researchers and subjects, and 3 single-blind studies [15,27,28]. In the description of the outcome, 7 studies (15,16,18,20,25,) used a blinding method, 1 (28) did not use any, and the rest failed to explain this. In terms of follow-up bias and reporting bias, all studies provided comprehensive information on follow-up or exclusion, while 5 studies (15,19-21,28) did not fully explain the selective reporting of research results, and 1 study (27) had repor- ting bias. For other biases, 3 articles (13,14,23) did not mention any.
EFFECT OF THE PROBIOTICS ON SERUM LIPID LEVELS
A total of 21 independent studies with 1429 subjects for changes in TC, LDL-C, HDL-C and TG were selected in this meta-analysis. It could be observed that probiotics could significantly lower TC (WMD: -0.34 mmol/L, 95 % CI: -0.45 to -0.23 mmol/L) and LDL-C (WMD: -0.26 mmol/L, 95 % CI: -0.36 to -0.17 mmol/L) levels in non-obese healthy adults with hyperlipidemia, while no significant effect between the probiotic intervention and control groups was observed on HDL-C (WMD: 0.00 mmol/L, 95 % CI: -0.02 to 0.02 mmol/L) and TG (WMD: -0.08 mmol/L, 95 % CI: -0.18 to 0.01 mmol/L) levels.
As regards heterogeneity, it was shown that TC and LDL-C had a higher heterogeneity (p < 0.001, I2 = 73.9 % and p < 0.001, I2 = 79.0 %), while HDL-C and TG had moderate heterogeneity (p = 0.001, I2 = 56.6 % and p = 0.003, I2 = 52.4 %). The detailed description results are shown in the forest map below (Figs. 4 to 7).
SUBGROUP AND SENSITIVITY ANALYSIS
Subgroup analyses were performed to evaluate the effect on baseline serum lipid levels, age, intervention duration and strains, form of probiotics, type of strains, and study type. It could be observed that the probiotics could significantly lower serum lipid levels in the TC and LDL-C groups when added in the yogurt or fermented milk, and the types of strain were Enterococcus and Streptococcus. It was also seen in the LDL-C group that probiotics could lower LDL-C levels when concentration is 3.4-4.1 mmol/L, and might be more effective on younger people (< 50 years old), with longer duration of treatment (> 6 weeks) and with Lactobacillus plus Bifidobacterium and Enterococcus plus Streptococcus. For the TG group, the heterogeneity might originate from intervention duration, the form of probiotics, and the type of strains. The detailed description results are shown below (Tables III to VI).
In order to explore the impact of each study on the stability of the combined results, a sensitivity analysis was conducted by using the one-study-removed approach. It showed that there was no significant change in heterogeneity and the combined effect size (CES) after combination of each trial in the TC group, which indicated the results were robust enough. For the LDL-C group, however, when the first period of Fuentes et al. (18) was removed, heterogeneity could decline greatly (I2 from 79 % to 45 %), while the CES did not change widely (WMD: 95 % CI from -0.26 [-0.36, -0.17] to -0.30 [-0.38, -0.23]). For the HDL-C group, there were two independent trials which could make a difference in the results. Both heterogeneity and CES changed when the second period of Fuentes et al. (18) was removed (I2 from 57 % to 0 %, WMD 95 % CI from 0.00 [-0.02, 0.02] to -0.01 [-0.02, -0.00]); heterogeneity changed but the CES was kept consistent when Park et al. (24) was removed (I2 from 57 % to 42 %, WMD 95 % CI from 0.00 [-0.02, 0.02] to 0.00 [-0.02, 0.03]). For the TG group, four independent trials could make the results unstable. The second period of Agerbaek et al. (13) and the first period of Jones et al. (20) had an effect on both heterogeneity and CES when removed (for Agerbaek et al. [13], I2 from 52 % to 41 %, WMD 95 % CI from -0.08 [-0.18, 0.01] to -0.11 [-0.19, -0.02]; for Jones et al. [20], I2 from 52 % to 49 %, WMD 95 % CI from -0.08 [-0.18, 0.01] to -0.10 [-0.19, -0.00]), while Ahn et al. (14) and Park et al. (24) only changed heterogeneity when removed (for Ahn et al. [14], I2 from 52 % to 47 %, WMD 95 % CI from -0.08 [-0.18, 0.01] to -0.06 [-0.15, 0.03]; for Park et al. [24], I2 from 52 % to 15 %, WMD 95 % CI from -0.08 [-0.18, 0.01] to -0.05 [-0.14, 0.03]). From that we found the heterogeneity of LDL-C might originate from Fuentes et al. (18), and the results of the HDL-C group and TG group were unstable.
PUBLICATION BIAS
Funnel plots (Figs. 8 to 11) and Egger's regression tests were performed to detect publication bias on the results of TC, LDL-C, HDL-C and TG. The first three funnel plots were visually symmetrical. Egger's linear regression tests indicated no significant publication bias for TC, LDL-C, HDL-C with a p-value equal to 0.259, 0.985 and 0.706. However, the TG group did show a publication bias, and it still existed after we used Duval and Tweedie's “trim and fill" method.
DISCUSSION
This meta-analysis included 16 (13-28) studies, which could be divided into 21 independent trials, and all studies included met the inclusion criteria. The results indicated that probiotics had a positive effect on the levels of LDL-C (-0.26 mmol/L) in non-obese healthy adults with hyperlipidemia. We performed a subgroup analysis, which showed that probiotics could significantly lower serum lipid levels in the TC and LDL-C groups when added to yogurt or fermented milk, and the types of strain were Enterococcus and Streptococcus. It also showed in the LDL-C group that probiotics could lower LDL-C levels when concentration is 3.4-4.1 mmol/L, and might be more effective on younger people (< 50 years old), with a longer duration of treatment (> 6 weeks) and with Lactobacillus plus Bifidobacterium and Enterococcus plus Streptococcus. As a result, these data indicated that probiotics could provide a promising way on serum lipid level in non-obese healthy adults with hyperlipidemia.
There had been studies (29-33) on the effect of probiotics in patients with hyperlipidemia before, but most of the studies did not consider the impact of obesity, which is a disorder of metabolism that can affect serum lipid. In addition, Pourrajab et al. (31) only used yogurt without considering a capsule. As a result, to our knowledge, this is the first meta-analysis aiming at non-obese healthy people with hyperlipidemia, and the results might be different when compared with the studies before. We observed that probiotics could lower LDL-C levels, which is different from the study by Deng et al. (29), whose results showed that probiotics could also modulate HDL-C levels without considering their impact on obesity. For heterogeneity, we found that there was larger heterogeneity in the TC and LDL-C groups, while there was moderate heterogeneity in the HDL-C and TG groups. To identify the source of heterogeneity, we did a subgroup analysis including baseline serum lipid levels, age, intervention duration and strains, form of probiotics, type of strain, and study type on the four groups.
We found that intervention duration, form of probiotics, and type of strain might drive the heterogeneity found in the TG group since said heterogeneity declined to less than 50 % in each group of the three subgroups. Longer intervention duration (> 6 weeks), probiotics in capsules, and Lactobacillus were shown to be more effective for lower concentrations of TG. For the LDL-C group, we observed that baseline serum lipid levels had a certain impact on the results for serum lipid levels of 3.4-4.1 mmol/L, and the same situation also occurred in the form of probiotics with yogurt or fermented milk, which might need more experiments to verify. The results also showed that the probiotics taken by people less than 50 years old had a stronger effect on the LDL-C group. We speculated that it might be related to the change of intestinal flora, since the intestinal flora in younger subjects was more active, which further affected the absorption and effect of probiotics. As for intervention duration, using probiotics for a longer time (> 6 weeks) might be more effective according to the results. Lactobacillus plus Bifidobacterium and Enterococcus plus Streptococcus showed a significant effect of lowering LDL-C levels. For single Lactobacillus, which is a kind of traditional probiotic strain, we observed that it might also have a positive effect of lowering LDL-C and TC levels, though it showed a higher heterogeneity in subgroup analyses, which needed a larger sample size to prove its specific effect. The design type of the study could also affect the results in all four groups. Subgroup analyses found that crossover studies had no effect on serum lipid levels, which might be explained as a methodological limitation, for the washout period of each study was different, and there was no guarantee that the impact of the previous intervention could be completely eliminated.
So far, there have been many researches on the mechanism of probiotics in reducing the serum lipid. Most of them are focused on cholesterol, which can be divided into three categories: 1) inhibiting the synthesis of cholesterol. Probiotics can produce non digestible carbohydrates, improve the level of short chain fatty acids, and block the synthesis of liver cholesterol. Probiotics can also inhibit cholesterol synthesis-related enzymes to reduce serum cholesterol concentration; 2) regulate cholesterol absorption and transport. Probiotics can not only combine cholesterol, but also transform cholesterol into other substances to mitigate its absorption in the intestine. Meanwhile, probiotics can also block the transport of cholesterol through coprecipitation or inhibition of transporters; 3) promote cholesterol decomposition. After cholesterol is converted into bile acid, probiotics will produce the bile hydrolytic enzyme to hydrolyze conjugated bile acid into free bile acid, which is therefore difficult to absorb by the small intestine and is discharged from the body. Probiotics can also increase the activity of cholesterol decomposing enzymes to increase cholesterol excretion (37-40). In animal experiments, probiotics can activate the transcription of related genes, promote the absorption of cholesterol, inhibit the process of fatty transformation, and promote the decomposition, absorption, and utilization of fatty acids (41). At present, the research at the genetic level is still uncertain and needs more experiments to be supported.
Compared with the previous meta-analyses (29-33), our study contains the following advantages: first, our study excludes the influence of other diseases, especially obesity factors, which are often ignored in these previous studies (29-32). So the results are more accurate and suitable for those who are healthy and non-obese with high serum lipid levels. Second, we have no time and language restrictions in retrieving articles, and the retrieval strategy is more comprehensive, covering all relevant articles as much as possible. Third, in the article screening process, our standards are stricter. Some studies adopted interventions which included other components that might affect serum lipids. These kinds of studies are excluded, and research designs that were not rigorous were also excluded, so the articles included are more accurate, high-quality and low-bias.
There are also some limitations in our study: First, there still remains a large heterogeneity in the results of TC, LDL-C and HDL-C, although a subgroup analysis was carried out, and the cause of heterogeneity remained to be found. In the sensitivity analysis it was found that Fuentes et al. (18) had a great influence on heterogeneity in the LDL group, and this may be the source of heterogeneity. However, the cause of heterogeneity was not found in the TC group. And the results of the HDL-C group and TG group were unstable, which means there were potential and important bias factors related to the intervention measures. Second, there still remained a publication bias in the TG group. We tried to use the “trim and fill" method, but the publication bias remained. It may be caused by heterogeneity or small sample size in this meta-analysis. Third, several studies have adopted a cross design, which may be more rigorous than the parallel design. However, it cannot fully guarantee that the washout period is sufficient, which may have an impact on the results. Finally, in some experiments, the sample size is not large enough so the results may be accidental, which requires a larger sample size to prove.
CONCLUSION
This systematic review and meta-analysis of randomized controlled trials showed that probiotics may provide a promising way to reduce serum lipid levels in non-obese healthy adults with hyperlipidemia. However, the specific effect still needs more clinical experiments to be proven. Also, the safety and adverse reactions of probiotics are worth considering.