INTRODUCTION
The incidence of obesity is rapidly increasing in the entire world. In 2016, the World Health Organization (WHO) estimated that more than 1.9 billion adults aged 18 and older were overweight (1). In Mexico, data showed that the prevalence of being overweight between 20 and 29 years old was 29.5 % for men and 25.3 % for women for obesity (2).
Obesity results from a disruption of energy balance that leads to weight gain and metabolic disturbances that cause tissue stress, inflammation, and endothelial dysfunction (3) involving various environmental and genetic factors (4). Gene-association studies have identified several genes that affect obesity-related traits (5) and have suggested that single nucleotide polymorphisms (SNP) account for around 30 % of BMI (body mass index) variance (6).
Chronic low-grade inflammation contributes to obesity-associated comorbidities, and adipose tissue is a major immunologically active organ contributing to this inflammation (8,9). Adipose tissue is metabolically active that can secrete a variety of adipokines and proinflammatory cytokines such TNFα and IL-1β in addition to anti-inflammatory cytokines like IL-10 (7).
Interleukin 32 (IL-32) is a proinflammatory cytokine that performs essential immune regulation. It is expressed by human peripheral blood mononuclear cells upon activation, induced in human epithelial cells by IFN-γ, and in natural killer cells by IL-12 plus IL-18 (9). IL-32 participates in inflammatory disorders such as rheumatoid arthritis, ulcerative colitis, multiple sclerosis, and cancer (10). Despite this characterization, the specific role of this protein in obesity remains to be determined.
The gene encoding IL-32 protein is located on chromosome 16p13.3 and contains six exons. Several SNPs have been studied in various diseases (5,11-13). The polymorphism rs45499297 is located in the promoter region of the IL-32 gene, characterized by a change from T to C. However, the association between this polymorphism and BMI, fat percentage, and lipid profile has not been reported. This study aimed to evaluate the role of IL-32 serum levels and the rs45499297 polymorphism in obese subjects. Another objective was to carry out an in silico analysis to investigate the interaction of this gene with factors that regulate its expression and analyze the network of this gene.
SUBJECTS, MATERIALS AND METHODS
STUDY PARTICIPANTS
This study was cross-sectional and included 333 subjects - 245 females and 88 males - all between 18 and 69 years of age. The participants were classified into the following groups: 22 underweight (BMI < 18.5 kg/m²), 188 normal-weight (BMI = 18.5-24.9 kg/m²), and 122 overweight/obese (BMI > 25 kg/m²) subjects. Additionally, we also classified them by percentage of fat. This interpretation was made considering gender and age as follows: for men, low (F% < 8), standard (F% = 8-19.9) and high/obese (F% ≥ 20); in women, low (F% < 21), standard (F% = 21-32.9), and high/obese (F% ≥ 33) (14). These guidelines yielded 16 low-fat, 153 standard-fat, and 164 high-fat/obese subjects.
Subjects were recruited from September 2017 to August 2019. The demographic and clinical characteristics of the studied population were recorded in an appropriate questionnaire. The exclusion criteria were based on subjects taking anti-inflammatory medications, having a history of autoimmune diseases, chronic alcohol usage, pregnancy, or women in lactation. All study subjects signed an informed consent form. The local ethics committee approved the study (6/2017-2018).
BIOCHEMICAL ANALYSIS
Blood samples were taken after eight hours of fasting and obtained from the antecubital vein in test tubes without anticoagulants. The serum was obtained by centrifuging at 3500 rpm for 20 minutes. An Abbott Aero Set autoanalyzer with the original kit was used to measure plasma glucose, total cholesterol, triglycerides, and HDL levels. The Friedewald equation was used to calculate LDL levels.
MEASUREMENT OF IL-32 LEVELS
We quantified serum levels of IL-32 by ELISA DuoSet (R&D Systems, USA). Briefly, plates were coated by 100 µL per well with Capture Antibody, then 100 µL of standard or sample was added per well, and the procedure was performed according to the manufacturer's instructions. The absorption was determined at 450 nm.
GENETIC ANALYSIS
DNA extraction was carried out from peripheral blood leukocytes using the Purelink Genomic DNA mini kit, following manufacturer's instructions. IL-32 SNP rs45499297 (T/C) was genotyped by the restriction fragment length polymorphism-based method, as previously described (12). Forward 5'-GATTGCTGAGACCAGTGA-3' and reverse 5'-TCTCTGAGCCCAGGAATG-3' primers were used to obtain a fragment of 445 bp. Amplification was performed with PCR conditions in a gradient thermocycler. The thermal profile was as follows: an initial denaturation step at 95 ºC for 3 min, followed by 35 cycles of denaturation at 94 ºC for 30 s, annealing at 62 ºC for 30 s, and an extension at 72 ºC for 45 s, with a final extension step at 72 ºC for 5 min. Amplified products were visualized by 6 % acrylamide gel electrophoresis.
We treated amplified products with the enzyme BamHI. The outputs obtained were as follows: the TT genotype presented two fragments, 306 bp and 139 bp. The TC genotype showed 445 bp, 306 bp, and 139 bp fragments. The CC genotype is a fragment of 445 bp. Amplified products were visualized by 6 % acrylamide gel electrophoresis and stained with silver nitrate.
STATISTICAL ANALYSIS
The characteristics of subjects were described using simple frequency, percentages, mean and standard deviation. After applying the corresponding normality tests, the association between study variables was performed using the chi-square test, Kruskal-Wallis test, or ANOVA. A p < 0.05 was considered statistically significant.
IN SILICO ANALYSIS
The investigation of the factors that regulate transcription of the IL-32 gene due to the effect of the rs45499297 polymorphism was carried out using the HaploReg (15), PROMO (16), rVarBase (17), and AliBaba (18) software.
To analyze the IL-32 gene network we used the COXPRESdb database (19) to obtain the genes coexpressed with IL-32. We used the first 50 genes and then constructed these genes' protein interaction network using STRING (20). KEGG pathway enrichment analyses of these coexpressed genes were carried out in the Database DAVID (21) and WebGestalt (22).
RESULTS
The demographic and biochemical characteristics of the study population classified according to their BMI and body fat percentage are shown in table I. As expected, the overweight/obesity group presented elevated glucose, total cholesterol, triglycerides, LDL-C, and VLDL-C levels. Overweight/obese subjects also presented lower HDL-C levels (p < 0.001). When separating subjects by body fat percentage, the high-fat/obesity group presented higher glucose, total cholesterol, triglycerides, LDL-C, and VLDL-C levels.
Table I. Demographic and biochemical characterization of the study population classified by BMI and fat percentage

*p < 0.05 compared with the underweight group or low-fat group; †p < 0.05 compared with the normal-weight group or standard group.
Figure 1 shows the analysis of serum IL-32 levels. Underweight subjects had lower levels of IL-32 when compared to normal-weight subjects (p = 0.035). Besides, IL-32 was lower in subjects with low fat than in those with a standard fat percentage (p = 0.025) and high/obesity groups (p = 0.026).
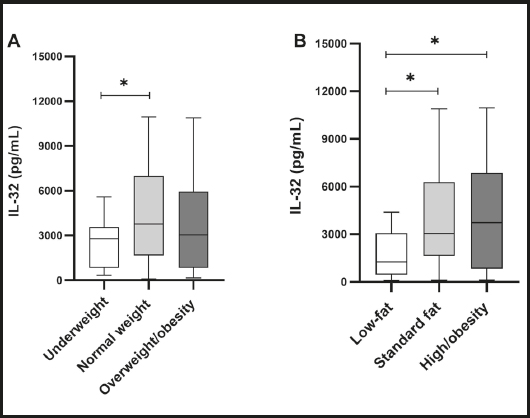
Figure 1. Analysis of serum IL-32 levels. A. Analysis according to BMI. The underweight group presented lower levels of IL-32 than the normal weight group. B. Analysis according to percentage of fat. The low-fat group presented lower levels of IL-32 than the standard-fat group and a high percentage of the high-fat/obesity group.
Later, we genotyped the IL-32 rs45499297 polymorphism. As shown in table II, the CC genotype is not associated with obesity, according to BMI. However, the TC genotype showed a statistical difference between the three study groups (p = 0.009); specifically, this difference was between the normal-weight and overweight/obesity groups (p = 0.003). We obtained similar results for allele C (p = 0.048); and, like in the genotype analysis, the difference was between the normal-weight and overweight/obesity groups (p = 0.024). The rs45499297 polymorphism analysis showed no significant differences between the different fat percentage groups for both genotypes and alleles (Table II). Also, the rs45499297 polymorphism did not affect biochemical parameters or IL-32 protein levels (data not shown).
Table II. Association of the IL-32 polymorphism by BMI and fat percentage and by gender
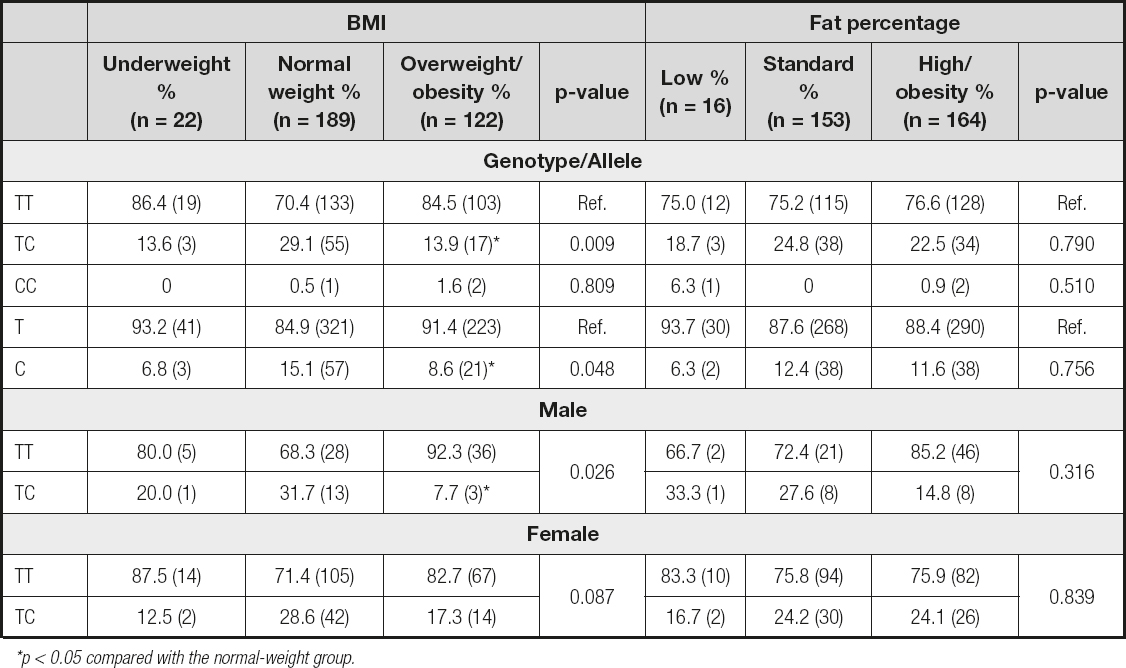
*p < 0.05 compared with the normal-weight group.
Table III. Transcription factors and motifs overlapping the rs45499297 IL-32 polymorphism according to different databases

We also analyzed the association between obesity and gender. Men have a higher frequency of obesity by percentage of fat (p = 0.007) and a trend towards higher BMI (p = 0.067) than women (data not shown). However, when we included genotype in this analysis (we omitted the CC genotype for statistical reasons), we found a statistical difference between the three groups (p = 0.026). Interestingly. it was observed that the TC genotype is less frequent in the male overweight/obesity group (p = 0.016). This suggests that the TC genotype may have a protective effect against obesity in young men. Data are shown in table II.
For the in silico analysis, we examined the potential functional effect of this polymorphism in various databases. As seen in table III, we found several transcription factors that overlap in the position of the rs45499297 polymorphism. It is noteworthy that two of them were inferred in three databases - Hepatocyte Nuclear Factor (HNF), with its variants. In addition, the transcriptional factor Specificity Protein 1 (SP1) was inferred in two databases. Therefore, this polymorphism appears to be a binding site for different transcription factors.
Based on the fact that IL-32 levels were decreased in the underweight group, we carried out the analysis of the IL-32 network (Fig. 2). We found that the 50 genes co-expressed with IL-32 were interconnected with 327 interactions. The top 5 of the KEGG metabolic pathways are shown in Figure 2A, along with the number of genes involved in each metabolic pathway. Figure 2B shows the network of genes co-expressed with IL-32. It should be noted that IL-32 does not participate in any of the enriched routes of the top 5.
DISCUSSION
IL-32 is a proinflammatory cytokine that is important for immune regulation and implicated in inflammatory disorders (11,23,24). We found significantly decreased levels of IL-32 in subjects who were underweight and low-fat when compared to normal-weight and standard-fat subjects. Fadaei et al. also performed IL-32 measurements and found higher levels in patients with type 2 diabetes mellitus versus controls, which supports the results from this study; in the diabetes group, they also found a positive correlation between BMI and IL-32 (25).
IL-32 production is predominantly induced by IL-1β, TNFα, IL-2, or INF-γ in monocytes and epithelial cells (23, 26). Interestingly, malnutrition presents alterations of IL-1β, TNFα, IL-2, and IFN-γ (27-30). Therefore, these cytokines may be regulating the production of IL-32 protein levels in underweight subjects. Rytter et al. found several levels of cytokines in children with malnutrition (27). Th1-cytokines IL-1, IL-2, IL-12, and INF-γ were lower in malnourished than in well-nourished children, while Th2-cytokines IL-10 and IL-14 were higher in those who were malnourished compared to those well-nourished. The authors conclude that malnourished children have an anti-inflammatory profile. Therefore, other altered cytokines in malnutrition may be affecting serum IL-32 levels in our study group.
We analyzed the rs45499297 polymorphism and its relationship with obesity for the first time in a Mexican population. We found that allele C and genotype TC of the IL-32 polymorphism are less frequent in the overweight/obesity group. Because so far there are no reports of this polymorphism in obesity or metabolic diseases, we cannot compare our results. However, Morsaljahan et al. analyzed this polymorphism in people from Iran with multiple sclerosis. They found that allele C was more frequent in patients than controls (11), so these results could indicate that this polymorphism is associated with another inflammatory disease.
We found that this polymorphism did not modify the biochemical parameters or serum levels of IL-32. There are also no reports of the rs45499297 polymorphism compared to biochemical parameters. However, in a study reported by Damen et al., which analyzed IL-32 promoter rs4786370 in patients with rheumatoid arthritis, the lipid profile was affected (12); this may be because IL-32 is an essential regulator of cholesterol transporters ABCA1 and ABCG1 (31). Therefore, other polymorphisms of IL-32 may be capable of affecting biochemical parameters, although it is advisable to look for new factors associated with the phenotype of obesity, as in this case the rs45499297 polymorphism.
The high/obesity group was comprised of more men than women. Other studies have reported results similar to ours, where young men have a higher percentage of obesity than women (32,33). However, our results appear to show that the TC genotype acts as a protective factor in men. This result could be due to the different hormonal profiles existing between both genders, as it is well known that people with obesity have high levels of leptin (34,35). Leptin is present in higher amounts in females than in males (36,37). Therefore, the TC genotype may confer protection against being overweight and obese in men more than women due to hormonal reasons.
Since it is known that transcription factors regulate gene expression by binding to the promoter region of the gene, it is presumed that the polymorphisms present in these regions affect gene expression. Hepatocyte Nuclear Factor 4 alpha (HNF4α), or its variants, and the transcriptional factor Specificity Protein 1 (SP1) were inferred in different databases. Previous studies reported that the IL-32 gene has a binding site for these transcriptional factors (39,40). However, we have inferred that the binding site overlaps with the rs45499297 polymorphism.
Our network analysis also showed the interconnectivity between signaling pathways such as human T-cell leukemia virus 1 infection (HTLV1), primary immunodeficiency, hematopoietic cell lineage, T-cell receptor signaling pathway, and NF-kappa B signaling pathway. Malnutrition has been associated with HIV and HTLV1 (39,40), so it is inferred that genes co-expressed with IL-32 are involved with these metabolic pathways, in addition to the fact that this cytokine is modified in malnourished states. This information is essential to understand disease mechanisms, especially in diseases with a high degree of complexity, such as obesity, in which several metabolic pathways are involved in the pathogenesis of the disease.
One limitation of the current study is the mean age of the participants, as the findings cannot be generalized to middle-aged or mature adults. So more research is needed with a greater range of ages. In addition, the results of the in silico analysis must be corroborated by experimental work.
CONCLUSION
Until now, there is little information on IL-32 and obesity. This study found an association of the rs45499297 polymorphism with overweight/obesity. However, there was no association of the polymorphism with lipid profile and serum IL-32 protein levels. The level of IL-32 was lower in underweight and low-fat subjects. On the other hand, the in silico analysis showed that this polymorphism is found in the binding site of several transcriptional factors. In addition to that, the enrichment of the metabolic pathways is diverse.
The results presented in this work could stimulate future research to clarify the pathophysiology of obesity, which has become a global problem that increases over time. Moreover, it is possible to better understand this pathology from a nutritional perspective and the genetic and immunology points of view.















