INTRODUCTION
Among nasoenteral tube (NET)-related complications, mechanical complications are most feared by healthcare teams because of the potential harm they may cause. Nevertheless, most studies on the subject have stuck to case reports (1-2) or retrospective study designs (medical record reviewing) (3-4), or have included a reduced number of observations (5). Absence of robust studies precludes, for instance, estimating the actual incidence and risk factors for these complications, also known as adverse effects.
Literature reviews have shown considerable variability in the incidence of NET-related mechanical complications (6-8). Accidental traction or removal of the NET is the most frequent one, with a reported incidence of 15.3 % (9), 43.5 % (5), and 55.7 % (10) in patients who received care in emergency services, nursing wards, and intensive care units, respectively. Obstruction rates have ranged from 9 % to 35 % in the literature (6). Inadequate positioning of the distal end of the NET has ranged from 0.3 % to 16 %, with insertion into the trachea, lung, or pleura being most commonly described (7). Injuries to the nasal mucosa or epistaxis have ranged from 1.8 % to 5 % after tube insertion (6,8), whereas the diet aspiration rate resulting from inadequate positioning of the NET was 0.6 % in patients admitted to emergency services (9) and 15 % in elderly people (11). None of the aforementioned studies assessed risk factors for NET-related mechanical complications.
This category of complications has consequences for patients, such as reductions in food intake, delays in medication administration, need for removal of the device, exposure of patients to new insertion procedures, and even severe adverse effects, including death (1-3,9-12). Therefore, it is an obstacle for patient therapy as a whole, and justifies identification of the reasons behind it and how frequently it manifests so that safety barriers can be established throughout the process. The objective of the present study was to evaluate the incidence of NET-related mechanical complications and the risk factors associated with them.
METHODS
The Strengthening the Reporting of Observational Studies in Epidemiology (13) guidelines were followed to develop a double cohort study. The data were collected in two stages: between June and November 2017 (cohort 1) and from May 2018 to May 2019 (cohort 2). The same research procedures were kept in both periods. The study was carried out in a teaching hospital in the Southern Region of Brazil with an 831-bed operational capacity, accredited by the Joint Commission International.
Patients who were 18 years old or older, admitted to clinical or surgical nursing wards with a NET already inserted, or who had it inserted at the hospital, were included. The device was a Dobbhoff 12FR tube. Patients who had been subjected to gastrostomy or jejunostomy, were confused and/or disoriented, were not able to consent to participate in the study, or were admitted to the hospital more than once were excluded.
Patients were selected by means of the electronic system that integrates the entire medical record by using prescriptions for feeding via NET as the starting point. A research assistant checked the list of patients who were using an enteric diet on a daily basis, aiming to include all potentially eligible patients, which reduced selection bias.
Before the study was initiated, the research team was trained, a process that was directly supervised by an experienced nurse. Agreement between observers was tested (14). The patients were monitored daily, from the beginning until discontinuation of nutrition administration via NET, transfer, hospital discharge, or death. The presence of one or more of the following mechanical complications was considered as the outcome: a) accidental traction or removal of the NET, caused by patients themselves or other people; b) obstruction of the NET (lumen blockage); c) inadequate positioning of the distal end of the NET (trachea, bronchi, lung, middle and distal third of the esophagus, or when the distal end of the NET turns toward the esophagus), as documented by an X-ray image; d) injury to the nasal mucosa with epistaxis; and e) aspiration of enteric diet as documented by an X-ray image or described in the medical record by the care team.
Other evaluated variables were: a) clinical characteristics of the patients, including underlying diseases, current diseases, Alzheimer's or Parkinson's or vascular dementia, psychomotor agitation, level of consciousness (assessed by using the Glasgow Coma Scale), presence of NET at hospital admission (designated by the researchers as “previous NET”), and the Charlson Comorbidity Index; b) medications administered via NET (pharmaceutical dosage forms and number of doses); c) NET care routine fulfillment checklist (flushing with water before and after administration of medications, after administration of nutrition, and every four hours; replacement of the nutrition equipment, precautions with infusion bombs; proper attachment of the tube; checking of the external length of the NET, that is, the distance between the proximal end and the point of insertion in the nostril, as a way of estimating if there was traction of the device; (d) presence of mechanical restraints in agitated or confused patients; e) presence of accompanying person; and f) number of patients per nurse.
The data were obtained by means of direct observation and by consulting medical records. They were recorded in electronic forms on the Google Forms® platform. Determination of the sample size followed Fletcher's recommendation (15) of including 10 outcomes for each variable in the multiple regression model. Therefore, it was not possible to evaluate the risk factors associated with the outcome for infrequent events.
The data were analyzed using SPSS® version 20.0 by observing the variables' characteristics and distribution. The incidence of NET-related mechanical complications was assessed by calculating the cumulative incidence [(number of events/total number of patients at risk)* 100] and incidence density [(number of events/total number of patient days of observation while at risk during study)* 1000], with their respective 95 % confidence intervals (CI).
After application of a univariate analysis, the Cox proportional hazards model was used to evaluate risk of traction or removal and obstruction of the NET, after it was fitted to time of exposure to the tube. The generalized estimating equations model was applied by considering each observation carried out with a patient as a sampling unit. The adopted level of significance was 5 % (p ≤ 0.05).
The methodological and ethical aspects of the proposal were approved (Certificate of Presentation for Ethical Evaluation no. 63247916.5.0000.5327), and all included patients consented to participate in the study.
RESULTS
The study followed 494 patients, totaling 3,676 patient days of observation over a median of 5 (3-10) days. Minimum age was 18 years and maximum age was 104 years, with most patients (69.4 %) being 60 years old or older. The most frequent reasons for admission were neoplasias (28.9 %) in structures in the mouth, pharynx, larynx, and esophagus (n = 70), stomach (n = 25), intestines (n = 13), and other sites (n = 35). The patients showed a median of 3 (1 to 4) comorbidities, with the maximum number of diseases described for the same patient being 12. Systemic arterial hypertension (45.1 %), smoking (41.7 %), alcoholism (22.7 %), diabetes (20.2 %), and stroke (11.5 %) were most frequent. The proportion of patients who were admitted to the hospital with a NET in (previous NET) was 16 %. The other characteristics are shown in table I.
Table I. Sample characterization. Data expressed as mean ± standard deviation, or median (25th percentile to 75th percentile), or absolute number (relative number)
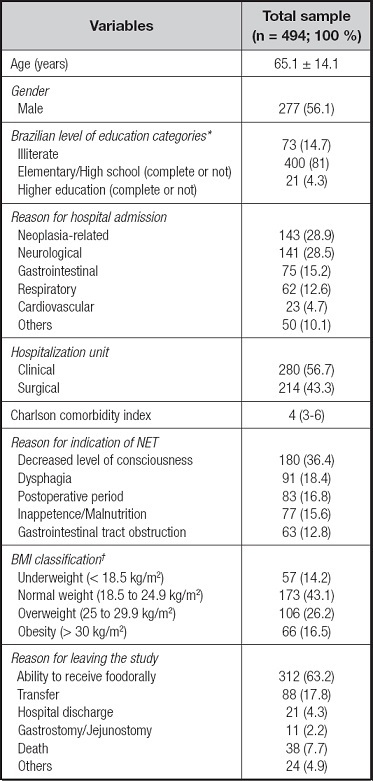
*Brazilian level of education categories: elementary school covers nine years, high school covers three years. †BMI: body mass index (considering the classification of nutritional status of adults). NET: nasoenteral tube.
†BMI: body mass index (considering the classification of nutritional status of adults). NET: nasoenteral tube.
Source: study data, 2020.
One third of the patients (n = 163) experienced accidental traction or removal of the NET, and obstruction occurred in 17 cases. Inadequate positioning of the distal end of the NET and bleeding in the nasal mucosa were found in only one patient each (0.2 % each). There was no record of food bronchoaspiration during the study period (Table II).
Table II. Cumulative incidence and 95 % confidence interval (95 % CI) of NET-related mechanical complications
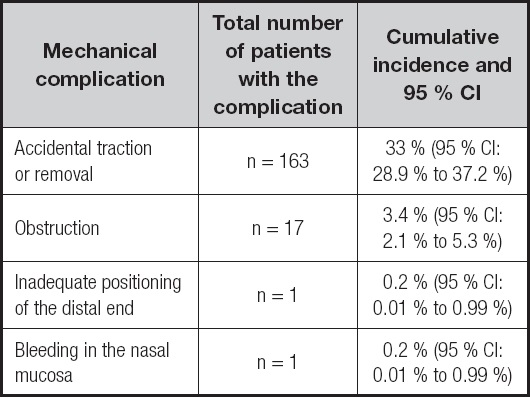
NET: nasoenteral tube.
Source: study data, 2020.
Over the 3,676 patient days of observation the researchers identified 273 episodes of accidental traction or removal of the NET. Most patients (n = 101) pulled out or removed the NET a single time. However, one of the patients experienced this event eight times. There were 24 cases of obstruction, and 5 patients had more than one obstruction episode (2 experienced the event 3 times, and 3 experienced the event twice). These data are shown in table III.
Table III. Incidence density and 95 % confidence interval (95 % CI) of NET-related mechanical complications
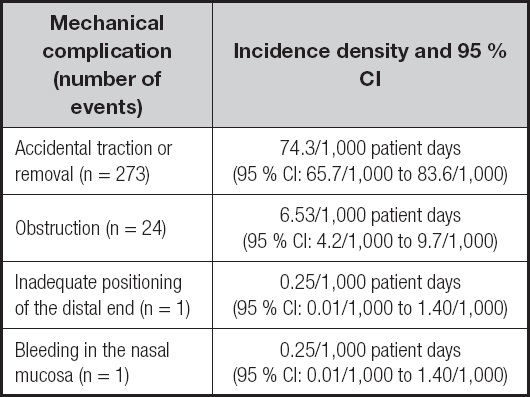
NET: nasoenteral tube.
Source: study data, 2020.
A history of stroke or neoplasia was independently associated with risk of accidental traction or removal of the NET, fitted for days of use of the device during hospitalization. The only independent risk factor for obstruction was use of the NET before hospital admission (previous NET), although the univariate analysis showed that the reason for indication of the NET “gastrointestinal tract obstruction” was statistically significant (HR: 6.69; 95 % CI: 1.67 to 26.8; p = 0.007) (Table IV).
Table IV. Cox proportional hazards regression analysis to evaluate factors independently associated with mechanical complications related to accidental traction or removal and obstruction of the NET
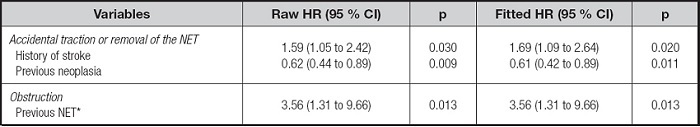
*Patient was admitted to the hospital with a NET in. NET: nasoenteral tube.
Source: study data, 2020.
The analysis of data about each observation day (patient days, with n = 3,676) indicated an increase in risk by 75 % for accidental traction or removal of the NET associated with a history of stroke. Each extra point on the Glasgow Coma Scale and each year in patient age led to an increase in risk by 9 % and 2 %, respectively. In contrast, each measurement of the external length of the NET was associated with an 11 % reduction in the risk of accidental traction or removal of the device. For NET obstruction, only one independent factor was found: the proportion of observations in which opioids in the form of pills were administered via the NET (Table V).
Table V. Analysis of generalized estimating equations with a binary logistic model to evaluate factors independently associated with the mechanical events of accidental traction or removal and obstruction of the NET
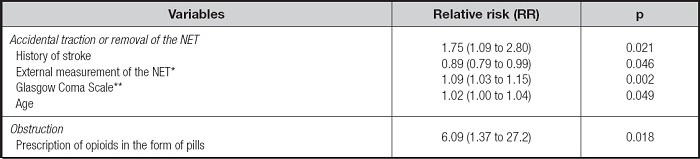
*The number of external measurements of the NET taken over 24 hours was assessed..
†Glasgow Coma Scale - score from 3 to 15; the average was calculated according to follow-up time. NET: nasoenteral tube.
Source: study data, 2020.
Only one case of inadequate positioning of the distal end of the NET (in which an imaging test showed that the distal end of the device was turned toward the esophagus), and one case of epistaxis secondary to insertion of the NET, were recorded. In both cases, the mechanical complications were not characterized as severe adverse effects, and the patients' condition improved, with neither increased length of hospital stay nor need for extra therapeutic measures.
DISCUSSION
The present study analyzed data from patients and daily observations of patients (patient days), and established that NET-related mechanical complications, especially traction and obstruction, were frequent. It also showed the risk factors associated with these two outcomes.
Few well-designed prospective studies have assessed the incidence of accidental traction or removal of a NET in patients admitted to nursing wards and the risk factors associated with these events. Only one multicenter study (16), carried out in seven Brazilian hospitals, evaluated the factors associated with NET-related mechanical complications in patients who used this device. The reported incidence of accidental traction or removal of the device (70.1 %), obstruction of the device (13.1 %), epistaxis (2.6 %), and bronchoaspiration (4.7 %) were higher than those found in the present study. The above-mentioned study identified an increase in the risk of NET-related mechanical complications, which also included pneumothorax, organ perforation, tube migration, injury caused by nasal pressure, and multiple attempts to insert the device in patients who required intensive care (OR = 4.72; 95 % CI: 1.43-15.52; p = 0.011). Sleepy patients showed a lower risk (OR = 0.24; 95 % CI: 0.10 to 0.61; p = 0.003). Although multicenter studies allow the inclusion of higher numbers of patients in samples and, consequently, of outcomes, they are heterogeneous regarding the routines of care provided for NET users because different procedures are adopted in different institutions. Additionally, grouping mechanical complications as a single outcome precludes the identification of the factors associated with each type of complication, which differ, as shown in the present study.
A history of stroke proved to be a risk factor for accidental traction or removal of the NET. Patients with this characteristic seem to benefit from enteral nutrition, temporarily or permanently, but the results indicated that they are more likely to experience accidental traction or removal of the device (17-20). The findings of the present study corroborated a study carried out in England that reported a high percentage of accidental traction or removal of the NET in a unit that provided care to patients who had had strokes (18). Over 5 months, 202 insertions of the NET in 75 patients were reported. Most (65 %) episodes of accidental traction or removal (n = 127) were caused by the patients themselves. In other cases, the device was removed for the following reasons: clinical justification (need to perform tests such as endoscopy, for example) (12.5 %); cough, nausea, or vomiting (9 %); resumption of oral nutrition (7.5 %); obstruction (2.5 %); and death (2.5 %). Accidental traction or removal of the NET in patients with a history of stroke also seems to be recurrent in other settings.
In clinical practice, there is a perception that the level of alertness or agitation of patients can contribute to increasing the chances of accidental traction or removal of the NET. The present study showed that the more alert and responsive the patients were, the higher the risk of accidental traction or removal of the device. Gimenes et al. (16) found a reduction by 76 % in the risk of NET-related mechanical complications in sleepy patients. Similar results were obtained, by applying a univariate analysis, in a study (9) in which patients were evaluated regarding incidents related to NET use in an emergency service. It showed that patients who were alert or agitated during NET insertion accidentally pulled out or removed the device more often than those who were calm or sleepy (78.3 % vs. 50.4 %; p = 0.014). Although the adopted methodologies and analysis strategies in the cited studies (9,16) were different from those used in the present study, it is suggested that the more alert or agitated the patients, the higher the risks of accidental NET traction or removal.
Surprisingly, previous neoplasia proved to be a protective factor for accidental traction or removal of the NET, reducing the chances of these events occurring in 39 % (after fitting for days of use of the device). No study that has identified this association was found. However, the literature often addresses the use of feeding tubes in patients with neoplasias, especially those affecting the gastrointestinal tract (21) as well as the head and neck (22-23). A study (22) showed that NET use duration reached 22 months. Another analysis indicated that patients with neoplasia used the device for a longer period (previous NET) than those exposed to contact with the device for the first time (50.6 % vs. 30.6 %, p = 0.001). It is possible to speculate that this protective effect could be related to the lengh of exposure to the device, with longer periods increasing patients' knowledge and self-care regarding the use of the device, including measures to minimize the risk of accidental traction or removal, even in inpatients.
Routinely measuring the external length of the NET was implemented in the setting of the present study with the objective of early identification of accidental traction or removal episodes. When this routine was observed, there was a reduction of 11 % in the risk of these events, which suggests that the practice is effective and should be disseminated in other care settings, as previously suggested by other researchers (24), as part of the care protocol.
The literature has described different conditions that lead to tube obstruction (25-30). They include device model and diameter (26-28), food viscosity (25), a slope lower than 45° in the position of the patient during and after nutrition administration (26), skipping flushing the device with water after infusion of food and medications (26), and use of oral (solid) pharmaceutical forms in the tube (25).
The incidence of obstructions found in the present study (3.4 %) was lower than that reported in other studies (16,25). Factors such as administration of water via the device, number of times the tube was flushed, number of doses of medication administered via the device, and use of infusion pumps, among others, were not associated with obstruction. However, the risk of obstruction was around three times higher for patients with previous NET and five times higher for those who had had opioids in the form of pills administered via the NET. Fitting the model for days of use of the device can partially explain the higher risk of obstruction, that is, the longer the time using the tube, the higher the risk of obstruction.
Other authors evaluated the effect of administering solid pharmaceutical forms on the obstruction of Dobbhoff 12FR tubes (25). The reported incidence of the event was 8 %. Patients who received food and medications concomitantly showed a higher risk of tube obstruction during the first 40 days of use of the device. Use of linagliptin (HR: 4.3; 95 % CI: 2.0 to 14.6; p = 0.001), nystatin (HR: 3.1; 95 % CI: 1.8 to 8.6; p = 0.001), rivaroxaban (HR: 2.4; 95 % CI: 1.4 to 6.2; p = 0.004), metformin (HR: 2.2; 95 % CI: 1.1 to 6.7; p = 0.048), and high-protein food (HR: 1.9; 95 % CI: 1.2 to 4.7; p = 0.010) were risk factors. It should be noted that the authors assessed obstruction-free time for patients who received solid pharmaceutical forms via the NET and those who did not. In the former group, this time was significantly shorter.
Many oral medications are not suitable for administration via feeding tubes, similar to some in viscous liquid form (31-34). Measures such as training teams regarding adequate preparation of drugs, diluting medication before administering (35-36), reviewing prescriptions, and standardizing medications to be administered via the NET (31-34) are among the most widely cited mechanisms to prevent obstructions.
As in all cohort studies, especially retrospective studies, only the variables that derived from the initial planning of the study were analyzed. Other variables that were not monitored, including the whole process of administration of medications via the NET, from preparation to administration itself, could explain at least some of the complications. However, the analysis covered a large number of other clinical and care routine variables that were prospectively monitored by a qualified research team, which allowed a better understanding of such things as the need to pay special attention to patients with a history of stroke.
It must be emphasized that the present study was intended to prospectively evaluate, not only the incidence of NET-related mechanical complications, but also the factors associated with accidental traction or removal and obstruction of this type of device by applying methodological rigor and a robust statistical analysis. One contribution of the present study that stands out is the finding, relevant to healthcare professionals, that accidental traction or removal of the NET is frequent, even in a hospital with a safety culture. The other mechanical complications, although less frequent, must be monitored, especially because of their potential severity. Additionally, the study provides healthcare teams with clues about patient characteristics that can be risk factors for mechanical complications.
CONCLUSION
The incidence of accidental NET traction or removal and obstruction found in the present study was similar to that reported in the literature: one-third of patients experienced accidental traction or removal of the device. Inadequate positioning of the NET's distal end and bleeding in the nasal mucosa were infrequent complications, and no episode of bronchoaspiration was recorded.
More attention should be paid to patients with a history of stroke, higher scores on the Glasgow coma scale, and/or older age, since these factors increased the risk of accidental NET traction or removal in the present study. In contrast, patients with neoplasia showed a lower risk of experiencing accidental traction or removal of the device, a finding that was not fully understood. Another factor that proved capable of reducing the chances of occurrence of these NET-related events was measuring the external length of the tube. Special attention must be paid to patients who are admitted to hospital with a NET already in because of another hospitalization, and those who receive opioids in the form of pills via the feeding tube, since these two groups showed an increased risk of NET obstruction.














