INTRODUCTION
Parenteral nutrition (PN) is a relatively complex life-sustaining therapy for patients with impaired gastrointestinal function and in other situations when oral or enteral nutrition (EN) are not tolerated or have to be avoided (1). Currently, clinical conditions expected to require PN in adult patients are intestinal failure, severe malnutrition, high-output intestinal fistula, chylous fistula, severe pancreatitis, gastrointestinal persistent or high risk of bleeding, and other conditions that contraindicate nutrition by other routes (1). The prevalence of PN varies depending on country, methodology and period studied. It ranged from 0.68 % to 0.82 % considering total hospital discharges (1,2), and from 7.9 % to 12 % considering only hospitalized patients at any given time (3,4). In intensive care units (ICU), a worldwide study reported that around 10 % of patients received exclusively PN during 2007-2013 (5). Overall mortality in adult hospitalized patients receiving PN is high and has been estimated at 15 % to 28 % (2,6). Several studies have assessed specific factors related to mortality in patients under PN like nutrient intake (7), hyperglycemia (8), previous EN withdrawal for gastrointestinal complications (9), duration of PN (10), bloodstream infection (11), inflammation-marker scores (12), weight loss (13), and the use of intravenous lipid emulsions (IVLE) containing fish oil (14). However, there is a lack of studies that assessed globally all factors related to mortality in a general cohort of adult patients requiring PN.
This study aimed to assess the factors related to mortality and their importance in a cohort of hospitalized adult patients who required PN considering their characteristics, type of admission, procedures, nutritional data, and adverse events during PN.
MATERIAL AND METHODS
STUDY DESIGN
This was a retrospective study performed in a 400-bed university tertiary hospital. All adult (≥ 18 years old) inpatients were eligible if they had received ≥ 4 days as first course of PN from January 2015 to December 2017 during their hospital admission. No other inclusion criteria were followed. Any subsequent course of PN was excluded from the study. Patients were also excluded if they received long-term (> 90 days) or home PN, or had not enough recorded data.
DATA COLLECTION
At the beginning of PN, we collected the data on patient demographics, main diagnosis, length of stay (LOS), anthropometric data (weight, height, body mass index [BMI], ideal body weight [IBW] (15), and previous unintentional weight loss), type of admission (emergent or elective), type of patient (medical or surgical), critically ill condition, severity of illness at the beginning of PN, comorbidity, nutritional risk, need for mechanical ventilation or renal replacement therapy. Severity was classified as minor (predicted mortality < 10 %), moderate (predicted mortality from 10 % to < 25 %), and major (predicted mortality ≥ 25 %) according to the Mortality Probability Model-III (16) at the beginning of PN. Comorbidity was classified as mild (predicted mortality < 10 %), moderate (predicted mortality from 10 % to < 25 %), and severe (predicted mortality ≥ 25 %) according to Exilhauser´s score score (17). Nutritional risk was classified as low (score ≤ 1), moderate (score = 2), and high (score ≥ 3) according to the Nutritional Risk Score (NRS) 2002 (18).
We also recorded serum levels of biochemical parameters at the beginning of PN: creatinine, calculated glomerular filtration rate (cGFR) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (19), albumin, prealbumin, lymphocyte count, C-reactive protein (CRP), bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP).
The nutritional data recorded were amount of protein and energy administered per kg of IBW, use of IVLE with fish oil, indication for PN, length of PN, days between admission and PN start, and if the patients presented at least one failed attempt at oral nutrition (defined as a reversion to clear liquids or “nothing-by-mouth” when the patient had begun to take solid food for ≥ 1 day) or EN (defined as a reversion to trophic EN or to stop EN when the patient had begun to progress to EN for ≥ 1 day).
Medication use for ≥ 3 days during PN administration was recorded for prokinetic agents (metoclopramide, domperidone, or erythromycin) and for potent opioids (morphine, fentanyl, sufentanil, or remifentanil).
Adverse events recorded during PN were number of days with hyperglycemia (days with at least one glycemia > 180 mg/dL) or hypoglycemia (days with at least one glycemia ≤ 70 mg/dL), new episode of sepsis (as reported in the medical history), appearance of an intestinal fistula, new episode of acute kidney injury (AKI) (cGFR ≤ 30 mL/min/1.73 m2), anastomotic suture dehiscence, emergence surgical intervention, and need for admission in ICU or PACU (postanesthesia care unit) for ≥ 3 days.
All patients were followed for at least 90 days after the end of PN or until death before 90 days. Mortality was extracted from hospital records, primary care records, and a central register of the regional health authority.
VARIABLES
The main variable was all-cause mortality at 90 days after the end of PN. Initial independent variables were anthropometric and demographic data, admission characteristics, severity, comorbidity, surgical procedures, biochemical parameters, nutritional risk, nutritional data, medications, and adverse events.
PARENTERAL NUTRITION
Overall, PN was designed to provide around 25 kcal/kg IBW/day and about 1.2-1.3 g protein/kg IBW/day. The composition of each PN was individually modified when necessary according to clinical conditions and laboratory parameters.
PN was prepared following usual hospital practices as an ‘all-in-one’ admixture and was administered in a 24-hour perfusion. All patients received the same products used to prepare PN: glucose solutions, standard amino-acid solution, vitamins, trace-element solution, and at least one of two IVLE: an olive oil-based IVLE or a multiple-source-oil IVLE containing 15 % of fish oil. This latter emulsion with fish oil was used mainly in patients severely ill or with moderate hypertriglyceridemia (triglyceridemia > 250-400 mg/dL). The former emulsion was used in the rest of patients.
STATISTICAL ANALYSIS
Continuous variables were reported as mean ± 95 % confidence interval (95 % CI), and compared using Student’s t-test; categorical variables were reported as frequency and percent, and compared using Fisher’s exact test.
A Cox proportional hazards regression model (CPHRM) was planned to analyze time-to-event data, with the dependent variable being days to death. The chosen approach was firstly to perform a univariate analysis of each independent variable to identify those significant to enter the multivariate model. Univariate analyses used the Kaplan-Meyer method with log-rank test for categorical variables and a univariate CPHRM for continuous variables. Independent variables initially tested were age, gender, emergent hospital admission, medical or surgical patient, critically ill at the beginning of PN, ICU admission during PN, need for mechanical ventilation or renal replacement therapy during PN, severity, comorbidity, nutritional risk, use of IVLE with fish oil, new episode of AKI or sepsis, emergence surgical intervention during PN, intestinal fistula, anastomotic suture dehiscence, use of prokinetic agents and potent opioids, number of patients with failed attempts of oral nutrition or EN, BMI, weight loss, protein and energy intakes, PN duration, days with hyperglycemic and hypoglycemic episodes, baseline values of cGFR, albumin, lymphocytes, bilirubin, and ALP. Variables with a p-value < 0.20 in the univariate analysis were initially included in the multivariate CPHRM. The final model was built using a backward elimination variable selection (LR).
The proportional hazard assumption for the CPHRM was tested by checking the Kaplan-Meier curves for crossing or dropping to zero for categorical variables, and by checking for significance when including time-dependent test variables in the model for continuous variables. Schoenfeld’s residuals global test was also calculated.
Values for p were two-tailed, and a p-value < 0.05 was considered statistically significant. Analyses were conducted using the SPSS version 25 (SPSS Inc., Chicago, IL, USA) and R version 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
All adult patients receiving PN during the study period were initially screened (Fig. 1). Finally, 634 patients entered the study and 140 (22.1 %) died. Of these, 60 (42.8 %) patients died during PN. Mean survival time for the entire cohort was 74.0 (95 % CI: 71.6-76.6) days. Mean time from end of PN to death was 17.6 (95 % CI: 13.5-21.6) days. Only 2 (0.3 %) patients were censored before 90 days of follow-up. Patient baseline characteristics are shown in table I. Patients were mainly surgical (471, 74.3 %). At the time of PN prescription the general distribution of patients by department was general surgery 236 (37.2 %), PACU 157 (24.8 %), ICU 123 (19.4 %), oncology 38 (6.0 %), and other departments 80 (12.6 %). Table II showed baseline biochemistry and nutritional parameters. Relevant complications, medications, glycemic control, and attempts at nutrition by another route are presented in table III.
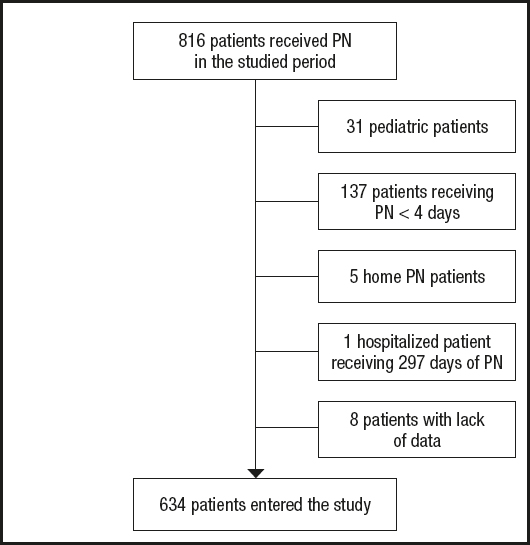
Figure 1. Recruitment procedure for patients in this study (68 (10.7 %) patients received more than one course of parenteral nutrition during the study period (PN: parenteral nutrition).
Table I. Baseline characteristics and main diagnoses.
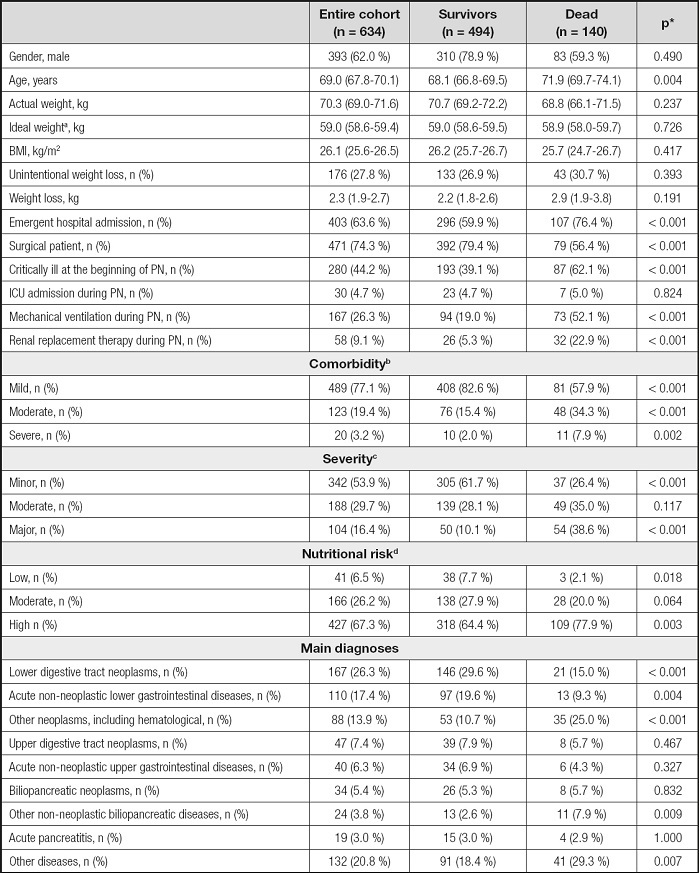
*p-values referred to the comparison between survivors and dead.
aCalculated by Miller’s equation (15);
bBased on Elixhauser’s score (17);
cBased on Mortality Probability Model-III at the beginning of PN (16);
dBased on Nutritional Risk Screening 2002 (18).
BMI: body mass index, calculated from actual weight; ICU: intensive care unit; PN: parenteral nutrition.
Table II. Baseline biochemistry and nutritional related parameters.
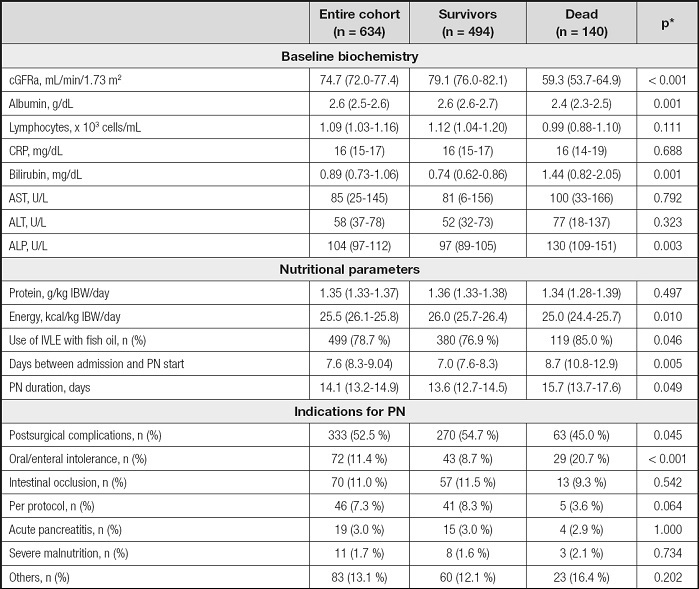
*p-values referred to the comparison between survivors and dead.
acGFR: calculated glomerular filtration rate by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (19).
ALP: alkaline phosphatase; ALT; alanine aminotransferase; AST: aspartate aminotransferase. CRP: C-reactive protein; IVLE: intravenous lipid emulsion; PN: parenteral nutrition.
Table III. Relevant complications, medications, glycemic control, and attempts of nutrition by other route during parenteral nutrition.
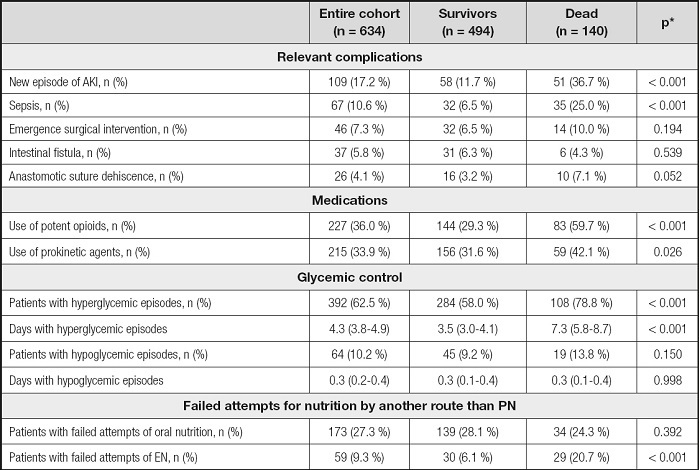
*p-values referred to the comparison between survivors and dead.
AKI: acute kidney injury; EN: enteral nutrition; PN: parenteral nutrition.
LOS was 37.7 (95 % CI: 35.3-40.2) days for the entire cohort, it being 38.4 (95 % CI: 35.4-41.4) days for survivors and 35.4 (95 % CI: 31.3-39.5) days for the patients who died (p = 0.323).
Categorical variables included in the multivariate CPHRM were emergent hospital admission, type of patient, critically ill at the beginning of PN, mechanical ventilation, renal replacement therapy, comorbidity, severity of illness, nutritional risk, use of IVLE with fish oil, new episode of AKI or sepsis during PN, emergency surgical intervention, anastomotic suture dehiscence, use of potent opioids or prokinetic agents, and patients with failed attempts at EN.
Continuous variables included age, weight loss, energy provided, days between admission and PN start, PN duration, days with hyperglycemic episodes, and baseline values of cGFR, albumin, lymphocytes, bilirubin, and ALP. All of them are presented in table IV.
Table IV. Univariate analysis.
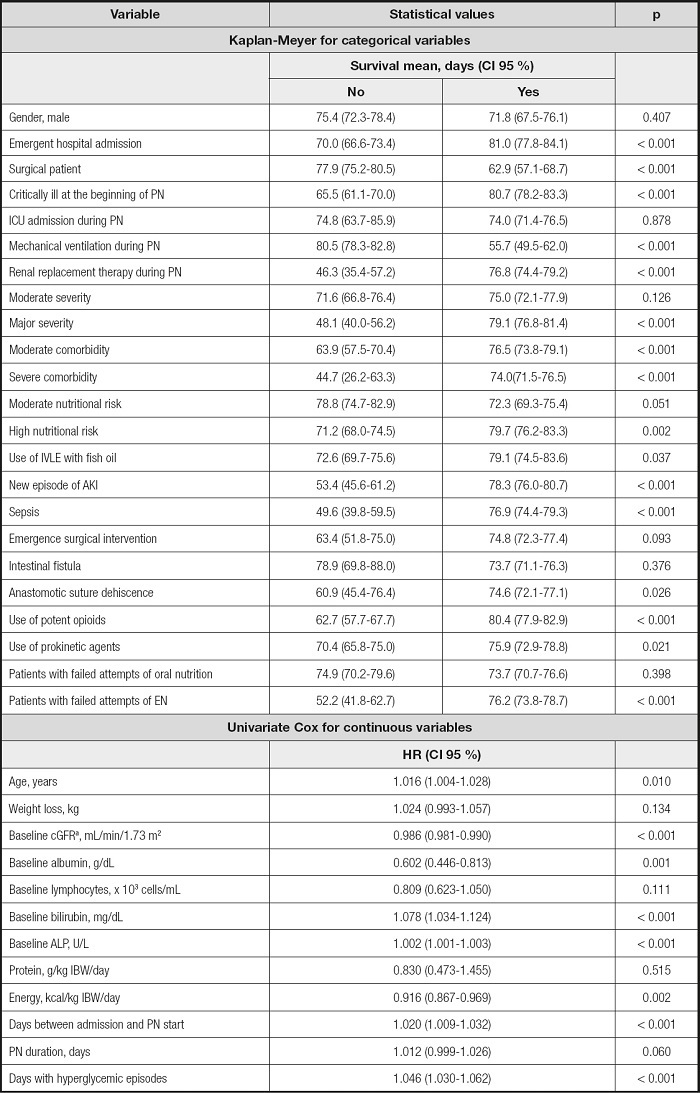
acGFR: calculated glomerular filtration rate by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (19)..
AKI: acute kidney injury; ALP: alkaline phosphatase; EN: enteral nutrition; IVLE: intravenous lipid emulsion; PN: parenteral nutrition
The final model included 14 variables that are shown in table V. The excluded variables are shown in table VI.
Table V. Multivariate Cox proportional hazard model: final model.
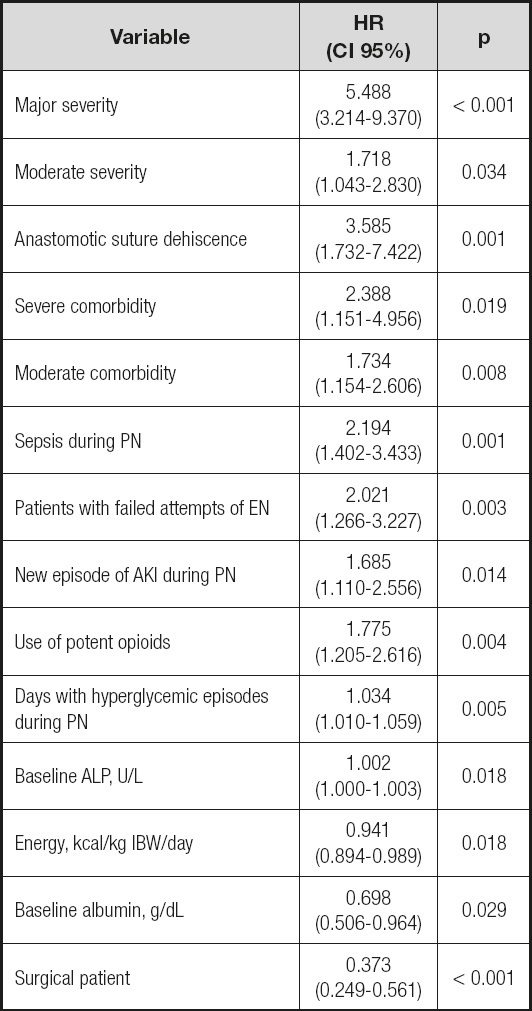
AKI: acute kidney injury; ALP: alkaline phosphatase; EN: enteral nutrition; IBW: ideal body weight; PN: parenteral nutrition.
Table VI. Multivariate Cox proportional hazard model: variables excluded in the final model.
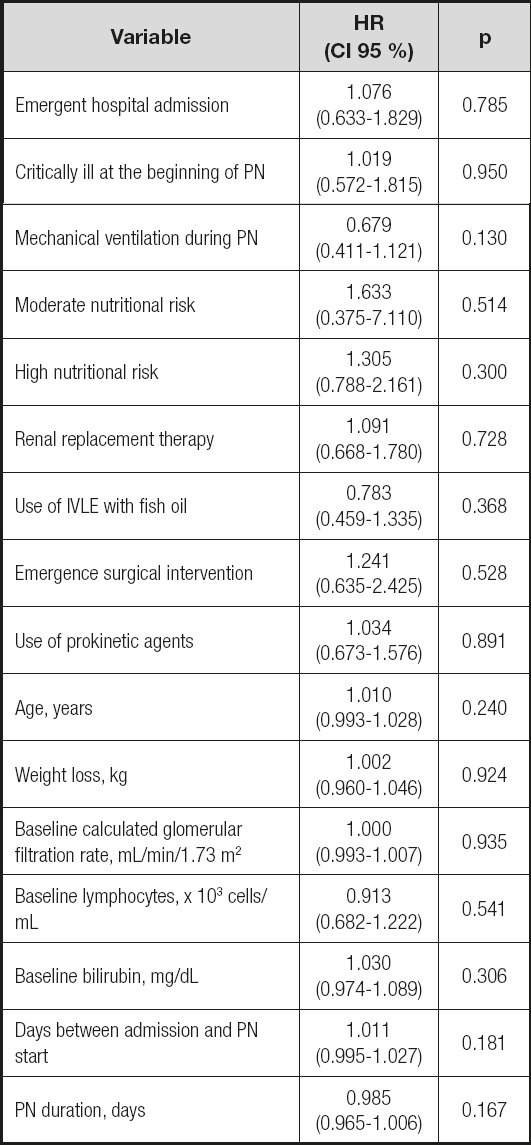
IVLE: intravenous lipid emulsion; PN: parenteral nutrition.
Kaplan-Meier curves for categorical variables neither crossed nor dropped to zero and time-dependent test continuous variables were statistically non-significant (data not shown). Schoenfeld’s residual global test showed a p = 0.326. Thus, the final model fulfilled the proportional hazard assumption.
DISCUSSION
Severity and comorbidity were the main factors related to mortality in the adult hospitalized patients receiving PN, but we also found some other factors not previously associated with mortality in these patients to our knowledge. Some of these factors may be of clinical relevance and could be modified to improve health outcomes in patients receiving PN.
Other obvious negative factors were new episodes of sepsis or AKI during PN. Even nowadays, sepsis has a high mortality rate (20), including in patients receiving PN (21). AKI has also been related to increased mortality in hospitalized patients (22). However, the effect on outcomes of an episode of AKI during PN have not been reported previously, to our knowledge. Anastomotic suture dehiscence is a severe surgical complication that increases morbidity and mortality (23). Our study was consistent with these previous findings.
Hyperglycemia has been associated with an increased risk of death among hospitalized patients receiving PN (8,24). In a large multicenter study, mean glycemia during PN > 180 mg/dL resulted in high risk of death during hospitalization (8). The prevalence of hyperglycemia in our study was higher than in previous studies as we used a stricter definition. Instead of considering mean glycemia, we considered a day with hyperglycemia each day with at least one glycemia > 180 mg/dL. In our model, each of these days increased the risk of death by about 3 %.
Serum albumin level is an independent predictor of outcomes in many diseases and health conditions. In hospitalized patients, hypoalbuminemia on admission increased mortality (25) and, for patients receiving PN, baseline hypoalbuminemia has also been associated with higher mortality (26). The results of our study agreed with these previous findings. Higher baseline albumin levels exerted protective effects on mortality.
In several studies, surgical patients presented lower mortality than medical patients especially in ICU settings (27-30). This lower mortality has been attributed to differences in underlying diseases, chronic health status, and the fact that surgical patients are routinely evaluated for perioperative risk to adjust anesthetic and surgical procedures (27,29). Additionally, surgical procedures may be considered “controlled” injuries and a subsequent postoperative ileus may require PN to maintain nutritional status when ileus is prolonged, but usually it may resolve uneventfully in several days. In contrast, medical patients usually suffer from “uncontrolled” injuries as neoplastic progression, infectious diseases, and other conditions that represent a higher risk to take their toll.
Increased levels of ALP have been associated with mortality in cardiovascular diseases (31) and in hospitalized patients (32). ALP is commonly used to assess liver function and hepatocellular injury, but it is also correlated with CRP and inflammatory processes (31). However, up till now, it was not a parameter usually associated with mortality in PN.
The use of opioids has been related to an increased risk of cardiopulmonary arrest (33), this being the most common cause of mortality in medical patients. Moreover, opioids cause gastrointestinal dysmotility (34) and prolong gastrointestinal dysfunction after surgery by their action on the mu-opioid gastrointestinal receptors (35). Thus, we may hypothesize that their use during PN could hinder the transition to oral or enteral nutrition and prolong intestinal failure.
Gastrointestinal failure leads to oral or EN intolerance, gastrointestinal hemorrhage, or ileus in an early stage, but it may be followed by extraintestinal disorders due to pathogenic crosstalk between the altered gut, circulating cells, and other organs developing or worsening systemic inflammation (36). This is of relevance for patients in severe conditions. Critically ill patients who presented EN intolerance had an increased mortality rate (9,37).
In our study, patients with failed attempts of EN could suffer from more severe gastrointestinal failure and, thus, be in a more severe condition than those who tolerated the transition to EN. It is noteworthy that none of the usual severity scoring systems include markers of gastrointestinal function. Patients with failed attempts at oral nutrition could be in a less severe condition and no effect on mortality resulted.
In critically ill patients, adequate nutrient supply is accepted to decrease mortality (7). Specifically in PN, protein doses of 1.2 g/kg/day have been shown to improve functional outcomes (38) and can reduce mortality in high-risk patients (39). Our results agreed with these findings although our nutritional supplies were slightly higher than those reported.
This study has several limitations. Firstly, its retrospective nature. Secondly, this was a single-center study. Thirdly, we did not consider the time of PN initiation (early versus late), which has been a subject of controversy in recent years. Lastly, we cannot discard other confounders that could modify our results. This has to be taken as a hypothesis-generating study to focus following studies on relevant factors potentially affecting mortality in hospitalized patients receiving PN.
In conclusion, factors related to mortality in hospitalized adult patients who required PN were mainly severity and comorbidities, but several other factors were also important, such as sepsis or AKI during PN, in addition to the use of potent opioids, hyperglycemia, and the energy provided. These latter factors could be modified to maximize outcomes in patients receiving PN. These factors suggest that PN requires accurate control and follow-up to maximize its benefits and reduce adverse events.














