INTRODUCTION
When vertebrates are born, a large number of microbiota parasite to the body through exposing external environment (mouth, vagina, skin, gut, etc.) (1). After a long time of evolution, a mutually beneficial symbiotic relationship has been formed between microorganisms and the host, in which gut microbiota are one of the most complex micro ecosystems (2). There are more than 100 trillion bacteria in the distal intestine, which are composed of thousands of microbiota, including two main category, Phyla Bacteroidea (60-65 %), Firmicutes (20-25 %) and some less Phyla Actinomycetes, Verrucous Microflora and Proteus, forming an interactive complex community (3). The main nutritional source of gut microbiota is the indigestible carbohydrate in the diet. Its fermentation produces acetic acid, propionic acid and butyric acid, called short chain fatty acids (SCFAs). SCFAs are secreted into the intestinal cavity and then enter the blood through epithelial cells, so as to reach various organs and play a role as the substrate of energy metabolism (4).
Gut microbiota have the functions of metabolism, immunity and intestinal protection. They are strong metabolic capacity and functional plasticity and this functional plasticity of gut microbiota depend on the development stage at a great extent (5). Studies have found that the microbial function promoted by exercise during the developmental period (6). The significant of microbial development in this period has also been revealed in the study of Germ-free (GF) mice. It was found that the anxiety behavior of developing GF mice in the elevated maze could be weakened by transplanting gut microbiota of normally fed developing mice, but not in adult mice (7). In addition, early administration of antibiotics can lead to an increase in body weight and fat in adulthood (8). The gut microbiota during development period play an important role through the host life and have high plasticity (9). Therefore, the developmental stage may be the key regulation stage of gut microbiota. It is very important to intervene the gut microbiota in the developmental stage, so as to have an effect on the whole organism.
Campbell et al. found that exercise has a unique effect on gut microbiota independent of diet in mice (10). In the study of human, it is found that the populations of Prevotella and Brevibacterium of professional cyclists are significantly higher than those of amateur cyclists, and are closely related to the training frequency (11). In addition, in the study of obese and thin people, it was found that 6 weeks of continuously exercise increased the concentration of SCFAs in thin people, but had no effect on obese people. After the exercise stopped, the changes of gut microbiota were reversible, indicating that this change is related to obesity, independent of diet, but depends on the continuously exercise (12). Evans et al. also confirmed that exercise can prevent obesity and induce changes in the percentage of major bacteria in obese mice fed with high fat (13). It was found that six weeks of voluntary wheel running also significantly increased the butyrate concentration in the cecum of rats, resulting in changing in the composition of gut microbiota (14). Other study found that even four weeks of voluntary wheel running can increase the number of Bifidobacteria and Lactobacillus, both of which are related to emotion and lean body components (15). A large amount of evidences showed that exercise has a significant impact on gut microbiota in adult, but the evidence of the impact of exercise on gut microbiota in the development stage did not be well studied. Therefore, in order to reveal the effect of short-term exercise on gut microbiota in developing animals, we designed the following experiments with voluntary wheel running exercise. In this study, we used 1-month-old mice with voluntary wheel running exercise. After 4 weeks of voluntary wheel running exercise, 16S rRNA was used to sequence the feces of developing mice.
MATERIALS AND METHODS
SUBJECTS AND HOUSING
Male KM mice (1-month-old) from Hunan SJA Laboratory Animal Co., Ltd. were selected. With the approval of the animal ethics committee of Jiangxi Science and Technology Normal University. All mice were weighed weekly, and had ad libitum access to food and water. The feed was dried national rodent feed and fed in a naturally ventilated animal room. The temperature was 24.0 ± 1.0 ºC and the humidity was 40 %-60 %.
VOLUNTARY WHEEL RUNNING
One-month-old male mice born on the same day were selected for the experiment, and the mice were randomly assigned to the control group and the running group at the beginning of the experiment, 15 mice in the control group and 15 mice in the running group, a total of 30 mice, were raised in 6 polyethylene cages (270 mm × 260 mm × 200 mm), each 5 mice, and a total of three batches of experiments were performed to ensure that the mice were not separated from each other at the beginning of the experiment. No significant difference. In order to reduce the impact of running wheels on the environment, running wheels were placed in the cages of two groups of rats, and the runners that could move freely were placed in the running group of rats in one cage, while the control group and runners could not move freely. Normal feeding (16) 4 weeks after sampling and testing.
FECAL SAMPLE COLLECTION
After 4 weeks of normal feeding, a stool sample was collected at 9 am on the first day after the exercise. During sampling, mice were placed in sterile glass cages. After defecation, mice were collected with sterilized tweezers at the first time, and feces were placed in 1.5 ml sterile spiral cap tube (USA scientific, FL), put it into liquid nitrogen for rapid freezing and store it at -80 ºC (16).
16S rRNA GENE SEQUENCING AND MICROBIAL COMPOSITION ANALYSIS
Samples were prepared for sequencing using established protocols (17). The previously stored stool samples were extracted with DNA using QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). After sample preparation, variable region (V3-V4) of 16S rRNA genes present in each sample was PCR-amplified with forward and reverse primers (F515/R806). The reverse primer is barcoded with an error-correcting 12-base Golay code to facilitate demultiplexing of up to 30 samples (18). Following purification and precipitation to remove PCR artifacts, samples were subjected to multiple sequencing on an Illumina HiSeq. Operational taxonomic units (OTUs) were picked using a “closed reference´ approach” (19). In brief, this approach takes sequenced reads and compares them to a reference database. A sequence is considered a “hit” if it matches some- thing in the reference database at greater than 97 % sequence identity. If an experimental sequence failed to match any member of the reference collection, it was discarded. Closed-reference picking is preferable to “de-novo” or “open-reference” picking in well characterized mice gut communities because the curated reference database acts as a filter; low quality or noisy sequences which get past the quality control steps, but do not actually represent novel OTUs, are eliminated. GreenGenes May 2013 version was the reference database used (20), and all sequence processing was done with QIIME v 1.8.0 using the UCLUST algorithm. Taxonomy and phylogeny were taken from the GreenGenes reference collection. The sequence was spliced by FLASH (Fast Length Adjustment of Short reads, v1.2.11), and the quality was further controlled by Quantitative Insights Into Microbial Ecology (QIIME). Rarefaction is a conservative approach that normalizes library size to prevent type I errors in a variety of techniques applied by QIIME. Recent literature has questioned the “statistical admissibility” of rarefaction in the context of differential abundance testing (e.g. ANOVA), but provide a superior method for only the basic two-way comparison. PCoA, supervised learning, and other methods perform poorly without rarefaction when sequencing depth differs between samples. To check that our selected rarefaction depth was not responsible for erroneous conclusions, these data were also rarefied at higher levels to check that patterns were not artifacts of low sequence coverage. The sparse curve and the species distribution bar graph were constructed by QIIME, the alpha diversity measures and beta diversity measures of the two experimental groups were calculated (16).
RESULTS
VOLUNTARY WHEEL RUNNING AFFECTED THE BODY WEIGHT OF MICE
The figure 1A showed that the weight of mice in the control group and the running group presented an upward trend. There was no significant difference in the weight of mice in the running group and the control group at the first three weeks. At the end of the fourth week, the running group weighed less than the control group (p < 0.05).
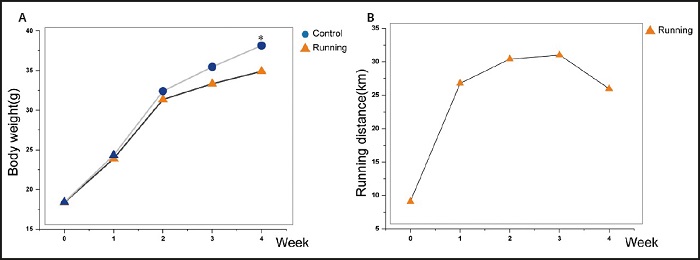
Figure 1. Comparison of exercise degree and mouse weight. A. There was no significant difference in body weight between the running group and the control group at 3 weeks, but there was a significant difference in body weight at the fourth week. B. The amount of exercise in the running group increased gradually in the first three weeks and decreased gradually from the fourth week.
EFFECT OF VOLUNTARY WHEEL RUNNING ON OUT STATISTICS OF GUT MICROBIOTA
To determine the composition of gut microbiota, the 16S rRNA sequencing technology were used. A total of 30 fecal samples (15 in the control group and 15 in the running group) were collected. Under 97 % similarity, 638 OUT were obtained and determined as the core microbiota of the running group and the control group. Through the rapid visualization of species composition and abundance, the microbiota was further analyze. At the same time, the number of OUT shared by the two groups of mice is 602, 25 were specific to the control group and 11 were specific to the running group (Fig. 2A). To evaluate the adequacy of sample number and species richness, the species accumulation box diagram was used. The number of samples reaches 30, the observed number curve of species in the figure tends to be flat, indicating that the experimental sample size is sufficient (Fig. 2B). The width and flatness of RANK curve reflected the higher richness and uniformity of species composition in this experimental sample (Fig. 2C).
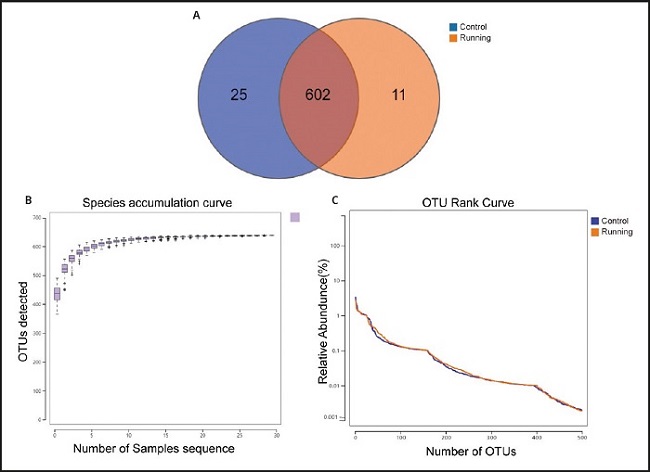
Figure 2. Comparison of abundance of gut microbiota between running group and control group. A. The two groups of mouse samples share the Venn diagram of OTU. B. Species accumulation box diagram shows species richness. C. Rank curves represent species richness and evenness. The curve width indicates that the species composition is more abundant. The flat curve indicates that the species composition is relatively uniform.
EFFECT OF VOLUNTARY WHEEL RUNNING ON OF GUT MICROBIOTA IN THE PHYLUM AND GENUS LEVEL
The species composition and proportion are intuitively displayed through the species column chart. In the phylum level, Firmicutes (55.80 %), Bacteroidetes (42.63 %) and Proteus (0.69 %) are the main flora of the gut microbiota in the running group. These three dominant flora account for 99.12 % of the total sequence, and unclassified gut flora only accounts for 0.88 %. In the control group, Firmicutes (54.02 %), Bacteroidetes (44.2 %) and Proteus (0.86 %), and unclassified intestinal flora accounted for 0.92 % (Fig. 3A). In the genus level, the most abundant genus in the running group included Clostridium Xlva (19.86 %), Barnesiella (18.05 %), Parabacteroides (5.59 %), Alistipes (5.21 %) and Intestinimonas (1.34 %).The most abundant genus in the control group included Barnesiella (23.02 %) and Clostridium XlVa (18.18 %), Alistipes (4.92 %), Bacteroides (4.15 %) and Intestinimonas (2.26 %). Although the gut microbes in the running group were slightly different from those in the control group among the 10 genus, but the significant difference caused by voluntary exercise was only found in Intestinimonas (p < 0.05).
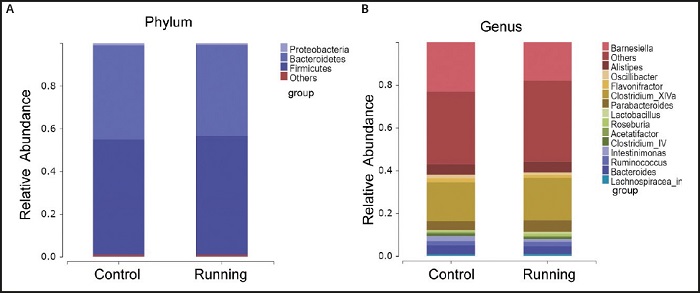
Figure 3. Comparison of gut microbial composition between running group and control group. A. Microbiota classification at the phylum level of running group and control group. B. Microbiota classification at the genus level of running group and control group (less than 0.5 % is classified to others).
EFFECTS OF VOLUNTARY WHEEL RUNNING ON GUT MICROBIOTA IN ALPHA DIVERSITY MEASURES
In order to further analyze the differences of microbial community structure between the two groups of mice, we carried out alpha diversity measures analysis, the sparse curve is flat. This indicated that the number of out clusters is reasonable (Fig. 4A). At the same time, through the comparison of various indexes, the Shannon index of gut microbial diversity in the running group is higher than that in the control group, but there is no significant difference (p = 0.14). At the same time, the ace index of the running group was also higher than that of the control group. In terms of gut microbial richness, there are trends in the sob index (p= 0.12), ACE index (p = 0.30), coverage index (p = 0.08) and Chao index (p= 0.74) of the running group were higher than those of the control group, but there was no significant difference between groups (Fig. 4B).
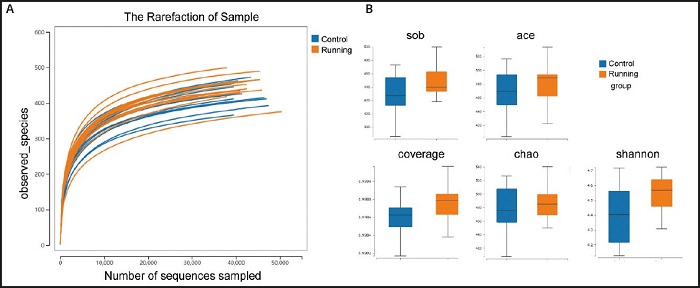
Figure 4. Comparison of gut microbiota between running group and control group in alpha diversity measures. A. The sparse curve is flat, indicating that the number of out clusters is reasonable. B. The comparison of alpha diversity, including species richness index (sob index, ACE index, coverage index, Chao index) and evenness index (Shannon index), all indexes of mice in the running group were higher than those in the control group.
EFFECTS OF VOLUNTARY WHEEL RUNNING ON GUT MICROBIOTA IN BETA DIVERSITY MEASURES
In order to visualize the similarity and uniqueness of gut microbiota in the running group and the control group, we used OTU principal component analysis based on unweighted UniFrac distance (PCA: PCa1 18.35 % vs. pca2 15.78 %) and weighted distance principal coordinate analysis (PCoA: pcoa1 17.34 % vs. pcoa2 13.33 %). In our study of gut microbiota in beta diversity measures. It showed that the significant separation (p < 0.01) between the gut microbiota of mice in the running group and in the control group (Fig. 5D). The non-metric multidimensional scale (NMDS) showed that there is difference of gut microbiota between running group and control group based on the Bray Curtis distance (Fig. 5C). Each component of circulating PCA is reached to maximize the interval between them. Therefore, in the analysis using PLS-DA, there is a significant difference between the running group and the control group. The two groups tend to aggregate separately from each other, indicating that each group has a unique microbial population (Fig. 5D). UPGMA cluster analysis was conducted according to the unweighted UniFrac distance matrix, and the relative abundance of species at the gate level was integrated. On the left was the result of UPGMA cluster tree and on the right was the species abundance histogram. It was found that the OTU of gut microbiota in the running group was in the same branch and close to each other. The gut microbiota of mice in the control group are in another branch and far away, indicating that there is a difference in the OTU composition of gut microbiota between the running group and the control group (Fig. 5E).

Figure 5. Comparison of gut microbiota between running group and control group in beta diversity measures. A: OTU principal component analysis (PCA: PCa1 18.35 % vs. pca2 15.78 %). B: Partial least squares discriminant analysis. C: Non metric multidimensional scaling. D: Principal coordinate analysis (PCA: PCa1 18.35 % vs. pca2 15.78 %), each point represents a sample, and there is a significant difference between the running group and the control group. E: Do UPGMA cluster analysis according to the unweighted UniFrac distance matrix, integrate the cluster results with the species relative abundance of each sample at the gate level, and show the species composition and differences.
EFFECTS OF VOLUNTARY WHEEL RUNNING ON FLORA OF GUT MICROBIOTA
In order to further determine whether specific individual bacterial taxa are rich in differences between the running group and the control group, we conducted Lefse analysis, which combines LDA with effect size measurement (Fig. 5A). The following five taxa show the differential distribution of LDA score > 2: parabactoids flora in the running group (p < 0.05) compared with the control group, the clostridiales flora (p < 0.01) and anaerovorax flora (p < 0.01) were more abundant in running group. Anaerotruncus flora (p< 0.05) and odoribacter flora (p < 0.01) were more abundant in the control group (Fig. 6A).
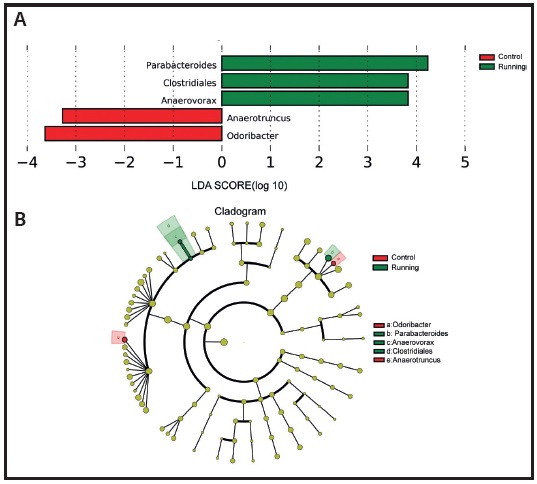
Figure 6. Ratio of LDA effect size of significantly different microbial groups derived from lefse analysis. A. Cladogram illustrates the phylogenetic relationship between the taxonomic groups of intestinal microbiota in the samples of mice in the running group and the control group. B. The absolute value of LDA is 2. The original p value of Kruskal Wallis test is used for discriminant analysis. The greater the score, the greater the difference.
The differential enrichment of specific bacteria was shown by the branching diagram based on LDA = 2. For pairwise comparison, two genera in the control group and one family and two genera in the running group play an important role. These results suggested that voluntary wheel running may have a significant impact on the shaping of gut microbiota in developing mice (Fig. 6B).
DISCUSSION
Exercise has a significant impact on gut microbiota (21), and may have a unique positive impact on the stage of development. In this study, we used voluntary wheel running for the first time to study the effect of voluntary wheel running on gut microbiota in the development stage of mice, so as to determine the effect of voluntary wheel running on gut microbiota in developing mice. The body weight, the statistics of OUT, the composition of microbiota and a and b diversity were measured. The weight of developing mice was decreased significantly, the gut microbiota b diversity was increased, and some microbial flora at the genus level and family level were changed after 4 weeks of voluntary wheel running compare to control group. These data showed that the development stage has a significant impact on gut microbiota after voluntary wheel running, and may have a series of positive effects.
Voluntary running is not only one of the more effective ways to affect the body in external stimulation, but also avoids the stress effects caused by other forced exercise (22). A recent study showed that there was a high correlation between physical and emotional stress during exercise and the changes of gastrointestinal microbial composition (23). For example, Clark and Mach had shown that exercise induced stress reduces the level of Turicibacter spp in the cecum and increases the level of Ruminococcus gnavus, which have a clear role in intestinal mucus degradation and immune function (24). In addition, exercise induced stress leads to the release of catabolic hormones, inflammatory cytokines and microbial molecules, and can affect the composition of gut microbiota through the brain gut axis (25). Therefore, the experiment selects voluntary exercise as the form of exercise to explore the effect of voluntary exercise on gut microorganisms in developing mice.
At the phylum level, Firmicutes and Bacteroidetes are the main flora. Firmicutes is an important source of butyrate and plays an important role in the metabolism, digestion and absorption of protein and other nutrients (26). It can not only act on energy metabolism and increase islet sensitivity, but also increase the expression of leptin gene. Bacteroides mainly produces acetate and propionate. Propionate had been shown to reduce the expression of fatty acid initial synthase, while acetate can promote the secretion of insulin and auxin releasing peptide (27). In a recent study, it was found that exercise not only prevented weight gain caused by high-fat diet, but also increased the proportion of Firmicutes/Bacteroidetes (28). The proportion of Firmicutes/ Bacteroidetes has been found to be related to animal models and human obesity status and predict obesity (29). Therefore, Firmicutes/Bacteroidetes is often regarded as a marker of obesity (30). However, the correlation between obesity characterization and proportion cannot be directly proved (31). This result has not been obtained in many studies, and even the opposite result has appeared, that is, the ratio of Firmicutes/Bacteroidetes decreases with the appearance of obesity characterization (32). In this study, it is found that when the weight measurement of developing mice is completed at the end of the experiment, The weight of mice in the running group was significantly lower than that in the control group (Fig. 1A), but the proportion of Firmicutes/Bacteroidetes in the control group was lower than that in the running group (Fig. 3A), which may be due to the large difference in the relative abundance of Firmicutes and Bacteroidetes in the same population (31).
At the family level, Lefse analysis showed that the clostridiales flora in the running group was significantly increased compared with the control group, and the decrease of clostridiales flora was related to the physiological imbalance of Crohn's disease, Crohn's disease is a disease of the digestive tract (33). In previous studies, it was found that regular and moderate running can have beneficial effects on gut microbiota, symptoms of irritable bowel syndrome and inflammatory bowel disease (34). In this experiment, after the effect of voluntary wheel running on developing mice, the abundance of clostridiales flora was improved (Fig. 6A). The results suggested that the effect of voluntary wheel running on developing mice may be caused by the changes of gut microbiota.
At the genus level, Lefse analysis showed that voluntary wheel running led to a significant increase in the abundance of parabacteroides flora and anaerovorax flora, and a significant decrease in the abundance of anaerotruncus flora and odoribacter flora. Parabacteroides flora, as one of the core flora of human body, is abundant in non-obese individuals (35), and some parabacteroides flora can produce propionic acid and succinic acid to prevent various metabolic disorders, such as insulin resistance and obesity caused by diet, which is negatively correlated with body weight (36). This relationship was also reflected in our experiment, that is, the weight of mice in the voluntary wheel running group decreased and the parabactoids flora increased. In addition, anaerovorax flora has therapeutic effects on a variety of diseases, such as the significant reduction of anaerovorax flora in patients with depression (37). In this experiment, exercise increased the abundance of parabacteroides flora and anaerovorax flora (Fig. 6A), indicating that voluntary wheel running may enhance physical immunity during development by increasing the abundance of parabacteroides flora and anaerovorax flora. Previous studies supported our experimental results and found that the abundance of anaerotruncus flora increased in obese mice (64) and humans (65). This suggests that anaerotruncus may be a marker of obesity. In this experiment, the anaerotruncus flora of mice in the control group was significantly more than that in the running group (Fig. 6A), and the weight of mice in the control group was significantly higher than that in the control group. In addition, voluntary wheel running may lead to inflammation through the increase of anaerotruncus flora abundance. The abundance of odoribacter flora is also related to a variety of diseases. The study found that the abundance of odoribacter flora in patients with attention deficit/hyperactivity disorder (ADHD) was significantly higher than that in healthy people, and the pathogenesis of ADHD was related to the decrease of dopamine secretion in the central nervous system, suggesting that it may be that odoribacter flora caused ADHD by regulating the secretion of dopamine (67). In this experiment, exercise reduces the abundance of odoribacter flora (Fig. 6A), which indicates that voluntary exercise may regulate the secretion of neurotransmitters in developing children, so as to prevent ADHD.
In this study, we found the effects of four weeks voluntary exercise on gut microbiota in developing mice and a series of beneficial effects. Through the changes of some families and genera of gut microbiota in developing mice, we can further understand how voluntary exercise has beneficial effects on gut microbiota in developing mice. It was found that short-term voluntary exercise had a specific effect on gut microbiota in the development stage. This experiment provides a theoretical basis and research basis for the effect of voluntary exercise on gut microbiota to promote health in the development stage, but the mechanism of action between exercise and gut microbiota and the beneficial effects of gut microbiota still need to be further explored.














