INTRODUCTION
Age-related macular degeneration (AMD) is the most common cause of irreversible vision loss in the elderly (1). AMD is a complex progressive disease, manifested in two different forms: wet (exudative neovascular) and dry (geographical atrophy). Exudative AMD is characterized by abnormal growth of new blood vessels, producing a central choroidal neovascular membrane (CNV), which in turn leads to retinal hemorrhage and exudation, and severe vision loss. With the increase in neovascular AMD patients worldwide, the burden of treatment is also getting heavier (2-4). Current studies have proved that nutritional factors, including antioxidants, carotenoids, lutein and zeaxanthin, and omega-3 polyunsaturated fatty acids, may have a protective effect on AMD (5-8). Long-chain omega-3 polyunsaturated fatty acids (omega-3 LCPUFAs) have anti-angiogenesis, anti-vascular proliferation, and neuroprotective effects on the factors and processes involved in the pathogenesis of proliferative and degenerative retinal diseases (9). In addition, they also have the ability to influence pathogenic factors and processes related to retinal neovascularization.
Omega-3 fatty acids include α-linolenic acid (ALA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), of which the latter two belong to the omega-3 LCPUFA group. ALA is an essential fatty acid that cannot be synthesized de novo and can be converted into omega-3 LCPUFAs (DHA and EPA). Importantly, long-chain omega-3 fatty acids also protect against oxygen toxicity, inflammation and age-related retinal damage, a key pathogenic process in retinal disease (9). The intake of long-chain omega-3 fatty acids is mainly provided by fatty fish. Several epidemiological studies have shown that the risk of AMD is negatively related to dietary omega-3 LCPUFAs and fish intake (5,10-15). But in the NET2 trial, the timing and incidence of CNV did not differ significantly between the DHA and EPA supplement groups and placebo groups (16). Similarly, in the AREDS2 study, the addition of DHA and EPA did not appear to have a protective effect on the progression of AMD (17). However, to our knowledge, there have been no systematic reviews and meta-analyses evaluating the association between omega-3 long-chain unsaturated fatty acids and exudative AMD. Therefore, we conducted an observational, experimental systematic meta-analysis to evaluate the protective effect of omega-3 long-chain unsaturated fatty acids on the progression of exudative AMD.
METHODS
This meta-analysis was designed, implemented, and analyzed in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) protocol and is reported following PRISMA guidelines.
LITERATURE SEARCH
Relevant literature was obtained by searching the Cochrane Library, PubMed, EMBASE and other databases, and relevant registered clinical trials were searched on Clinical Trials.gov. Search time ranged from the establishment of the database to August 31, 2021. Omega-3 Fatty Acid, Docosahexaenoic Acids, Eicosapentaenoic Acid and Age-Related Macular Degeneration were selected as subject terms, and then the respective free words were searched without study type, language, or national boundary limitations. Two researchers (XTM and YYS) participated in the whole process, and a third party participated in the discussion if there was any disagreement.
SELECTION CRITERIA
Our meta-analysis included selection studies that met the following criteria: 1) all observational studies, including prospective cohort studies, case-control studies, and cross-sectional studies; 2) for the exposure to dietary omega-3 long-chain fatty acid intake, the result is the incidence of neovascular AMD, providing an effect size (ES, including odds ratio [OR], hazard ratio [HR], relative risk [RR]) estimates and 95 % confidence intervals (CI), which compares the highest quartile or quintile of intake with the lowest quartile or quintile; 3) appropriate statistical techniques for adjusting key confounding factors such as age and smoking; 4) if the same data is used in multiple publications, the most recent complete study is included in our analysis. Exclusion criteria: reviews, meta-analyses, case reports, non-human studies, studies lacking adequate data, and other non-relevant publications.
DATA EXTRACTION
Two reviewers (XTM and YYS) were selected to independently search 4 databases, including grey literature databases (unpublished work, such as conference abstracts). The reference list of retrieved related publications and recent review articles was checked to supplement our search. The title and abstract were initially screened. Complete manuscripts were obtained for all potentially relevant studies, and then the full text of qualified studies was evaluated. Any differences were resolved through group discussion. The following information was extracted from each included publication: first author, year of publication, study location, study name, gender and age of participants, sample size (cases and number of participants), method for assessing dietary fatty acid intake, dietary fatty acid category, and ES estimates for 95 % CIs. If multiple multivariate adjustment models were used in the study to report risk estimates, the model with the most adjustment was extracted.
DATA SYNTHESIS
We used the RevMan software for the meta-analysis. Fully adjusted ES (OR, HR, RR) was used for each study. The standard error of the natural logarithm (ln) of ES was calculated from the 95 % confidence interval (CI) using the following formula: ln[upper limit of CI] - ln[lower limit of CI]) / 3.92. Heterogeneity between studies was tested using I2 statistics. When there was no significant heterogeneity, fixed-effects models were used to summarize the results. When substantial heterogeneity was detected (I2 > 40 %), random effects models were used and possible causes were explored; otherwise, mixed effects models were used.
RESULTS
STUDY EVALUATION
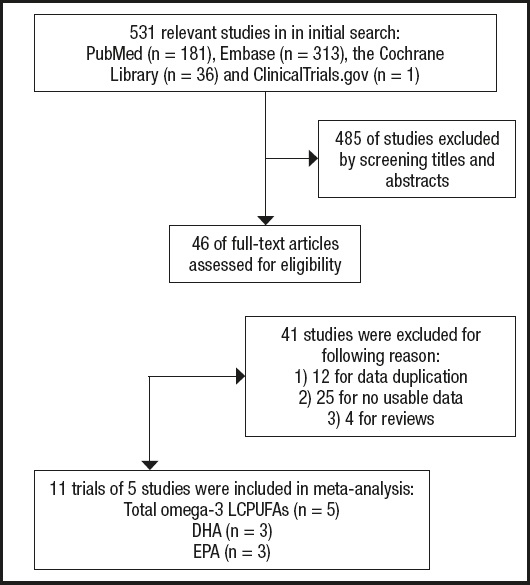
The search returned a total of 531 publications. Of these, 485 publications were excluded because they did not meet the predefined inclusion criteria. A total of 46 studies were evaluated for potentially eligible articles, and after reading the full text, 41 studies were excluded because of reviews, duplication of research data, and unavailability of research data. It is worth noting that in the Elvira Agrón 2020 study (7), two experimental data were included: AREDS and AREDS2, but due to the design of the study, half of the AREDS2 participants received DHA and EPA supplements, which caused confounding factors for the results, so they were excluded. Two researchers participated in the whole process, and a third party participated in the discussion if there was any disagreement. Ultimately, 5 studies reporting dietary FA intake were included in our meta-analysis. See detailed flow chart 1 below (Fig. 1).
STUDY CHARACTERISTICS
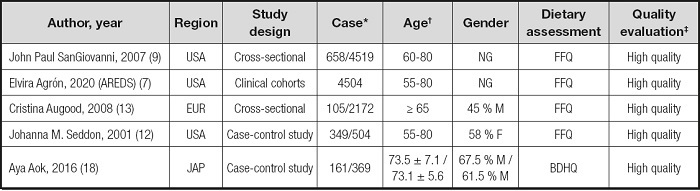
Table I summarizes the descriptive characteristics of the included studies. We included a total of 5 studies with a total of 12,068 participants. Three of the studies were conducted in the United States, one in Europe, and one in Japan. Five studies provided data on dietary FA and wAMD, including two cross-sectional studies, two case-control studies, and one cohort study (Table I).
Table I. Characteristics of the included studies.
NG: not given.
*Case (sample): cohort size or control.
†Age: mean (± SD) or range.
‡The Newcastle-Ottawa Scale (NOS) was used as the standard to evaluate cohort studies and case-control studies. Cross-sectional studies were evaluated using criteria recommended by the Agency for Healthcare Research and Quality (AHRQ).
QUALITY OF OBSERVATIONAL STUDIES
The Newcastle-Ottawa Scale (NOS) was used as the standard to evaluate cohort studies and case-control studies. Cross-sectional studies were evaluated using criteria recommended by the Agency for Healthcare Research and Quality (AHRQ). The included studies were of high quality (Table I). The food frequency questionnaires (FFQs) and brief-type diet history questionnaires (BDHQs) used in these studies to assess dietary fatty acid levels are applicable to large cohorts and provide information on a variety of foods. However, these tools have many limitations, including dietary misreports, which can lead to misclassification of dietary intake and/or portion size.
DATA ANALYSIS
Five of the included studies analyzed total omega-3 LCPUFA intake and wAMD risk (ES). Compared with the lowest category, patients in the highest category had a 49 % reduction in the risk of wAMD associated with dietary total omega-3 LCPUFA intake (ES: 0.51, 95 % CI [0.34, 0.75], I2 = 70 %, p = 0.01) (Fig. 2A).
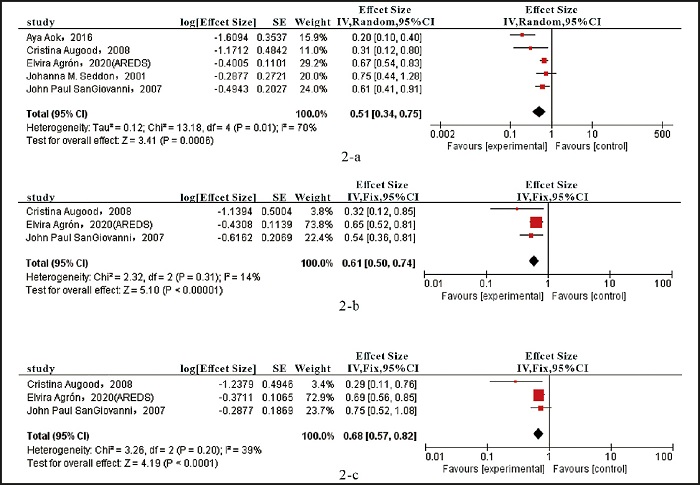
Figure 2. Forest plot of the effect size (ES) of wAMD comparing the highest with the lowest dietary intake categories. A. Total omega-3 LCPUFAs. B. Docosahexaenoic acid. C. eicosapentaenoic acid.
Three studies were included to assess DHA and EPA intake and wAMD risk (ES) separately. Compared with the lowest DHA consumption, the highest DHA consumption was associated with a 39 % lower risk of disease (ES: 0.61, 95 % CI [0.50, 0.74], I2 = 14 %, p = 0.31) (Fig. 2B). Compared with the lowest EPA consumption, the highest EPA consumption reduced the risk of wAMD by 32 % (ES: 0.68, 95 % CI [0.57, 0.82], I2 = 39 %, p = 0.20) (Fig. 2C).
In the correlation between total dietary omega-3 LCPUFA intake and wAMD, all 5 studies reported a negative correlation. However, there was significant heterogeneity in the results (I2 = 70 %, p < 0.001) (Fig. 1). For studies of the association between DHA and EPA intakes alone and wAMD, the highest and lowest point estimates of intake of this fatty acid are shown in figure 2A. One cohort study and two cross-sectional studies were included in the pooled analysis, all of which reported negative associations. The results were consistent across all studies (I2 = 14 %, p < 0.00001; Fig. 2B. I2 = 39 %, p < 0.0001; Fig. 2C).
ASSESSMENT OF REPORTING BIASES
There were insufficient numbers of studies to carry out a funnel plot analysis to investigate the relationship between treatment effect and study size.
HETEROGENEITY ANALYSIS
For the wAMD risk associated with the intake of total omega-3 LCPUFAs (ES: 0.51, 95 % CI [0.34, 0.75], I2 = 70 %, p = 0.01) (Fig. 2A), there was high heterogeneity. This heterogeneity mainly comes from the research of Aya Aoki et al. (18). Excluding this study, the risk of wAMD associated with total omega-3 LCPUFA intake was ES: 0.65, and the remaining four studies were consistent (95 % CI [0.54, 0.77], I2 = 0 %, p = 0.43) (Fig. 3). Compared with other studies, the demographic characteristics of Aya Aoki et al. are significantly different. The population studied is Japanese. And because of Japan's special dietary habits —fish intake is higher than that of other developed countries— cohort studies have shown large geographic differences in total fish intake, fish subpopulations, and number of fish species (19). As a result, their intake of omega-3 LCPUFAs is much higher than that of other studies. In addition, the brief Type Diet History Questionnaire (BDHQ) was used to evaluate dietary fatty acid intake in their study, unlike the Food Frequency Questionnaires (FFQ) used in other studies. The above reasons may be the important factors for its heterogeneity.
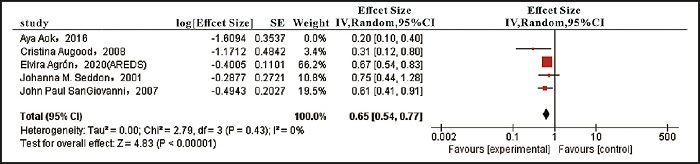
Figure 3. Excluding the research of Aya Aoki et al., forest plot of the effect size (ES) of wAMD comparing the highest with the lowest dietary intake of total omega-3 LCPUFAs.
There was no significant heterogeneity in the studies regarding the risk correlation between DHA and EPA and wAMD.
DISCUSSION
The main results overview provided evidence of a protective association between dietary omega-3 LCPUFA intake and incidence of wAMD. Two case-control trials, two cross-sectional trials and one cohort study were included in this meta-analysis. The trial divided 12,068 AMD patients into 4 or 5 groups based on their fatty acid intake, compared the highest intake group and the lowest intake group, and recorded the ES value (including OR and HR) and its 95 % confidence interval. A protective association was observed between dietary total omega-3 LCPUFA intake, DHA intake, EPA intake, and the incidence of wAMD.
Despite the heterogeneity introduced by the study by Aya Aoki et al. (18), all five studies showed a negative association between total omega-3 LCPUFA intake, DHA intake, EPA intake and wAMD incidence. It may mean that omega-3 long-chain fatty acid intake can have a protective effect on wAMD in different groups of people (including different races and dietary habits).
Although studies have shown a protective association between dietary omega-3 FA and early AMD, some studies have also shown that circulating omega-3 fatty acids are associated with a low risk of neovascular age-related macular degeneration (20-22).
However, the research by Zhong et al. (23) found that higher dietary omega-3 polyunsaturated fatty acids did not reduce the risk of advanced AMD, which is consistent with a previous randomized controlled trial report that omega-3 FA supplementation did not affect the risk of neovascular AMD. However, these findings are inconsistent with our meta-analysis of observational studies of neovascular AMD, which may be due to the fact that only a small proportion of early AMD cases are likely to progress to advanced AMD during the 5-year follow-up period (24). Therefore, the relatively low incidence and short follow-up period of advanced AMD may bias the results of randomized controlled trials.
Exudative AMD seriously affects patients' quality of life and reduces social productivity. Dietary adjustments to reduce the risk of AMD or slow its progress are of great significance for future research. This meta-analysis provides evidence for the protective effect of dietary omega-3 LCPUFAs on neovascular AMD in observational trials. Randomized controlled trials with a longer follow-up period may be needed in the future.