INTRODUCTION
Drug administration through a feeding tube (FT) —performed when oral feeding cannot take place— gives rise to various complications including gastrointestinal intolerance, metabolic or infectious complications, and mostly mechanical complications such as FT obstruction resulting from inappropriate drug administration (1).
Most patients receiving nutritional support through FT suffer from chronic diseases that require long-term pharmacological therapy. Administration of crushed medications is the most common cause of FT obstruction (2), resulting in the need to replace the tube and, in turn, increasing patient morbidity and associated health care costs (3). In addition, tablet crushing alters the mechanism of extended-release of enteric coating formulations, leading to a rapid increase in drug bioavailability (and a greater risk of side effects) (4) as well as to lower blood levels towards the end of the dosing interval (early reoccurrence of symptoms).
Factors affecting drug therapy via FT that should be considered include:
− Pharmaceutical form: current guidelines recommend, whenever possible, the use of liquid pharmaceutical forms as they hardly require any manipulation (5,6). Regarding the use of extended-release tablets, the way these pharmaceutical forms act is altered when crushed (5).
− Administration techniques: administration techniques may vary depending on the type of drug and its pharmaceutical form. Furthermore, the type of diluent used will differ according to drug bioavailability.
− Tube access: access site determines drug absorption and, as the pH in the jejunum becomes more alkaline, this may limit absorption of certain drugs (7). Changes in pH in the digestive tract also affect the degree of drug ionization and therefore the percentage that can be absorbed. Another important factor to consider is osmolarity as hyperosmolar formulations lead to faster transit rates if administered directly in the small intestine (8).
A study published by Givens et al. (9) showed that FT-related complications accounted for almost half of the visits to emergency departments in patients with advanced dementia. It is therefore necessary to review and adapt medications to this route of administration to avoid errors in drug administration (10). In addition, patient and/or caregiver education on how to administer medication, with appropriate monitoring and follow-up systems improving treatment management, are essential.
Regarding the pharmacist’s role, a study by Sánchez et al. (11) on pharmaceutical care in patients with enteral nutrition shows that the inclusion of a pharmacist in nutritional support teams helps resolve problems derived from the administration of drugs through FT. Pharmacists have the required knowledge enabling them to assess both nursing staff and patients/caregivers on the availability of appropriate preparations for enteral administration, interactions between drug-enteral nutrition, and potential adverse effects and incompatibilities (12-14).
The aim of this study was to assess a medication adaptation pathway for FT administration followed by clinical pharmacists for patients at discharge. We also aimed to assess and analyse the level of physicians’ acceptance of recommendations issued by the pharmacists in pharmaceutical care reports.
METHODS
We conducted a one-year retrospective, observational study at the 350-bed Costa del Sol Hospital in Spain, which provides care to a population of 410.022 inhabitants. The patient population consisted of all patients aged 18 years and older discharged from hospital during 2019 who had an FT inserted for the first time. These patients were monitored for six months following study inclusion, and patient follow-up visits for pharmaceutical care after inclusion were recorded.
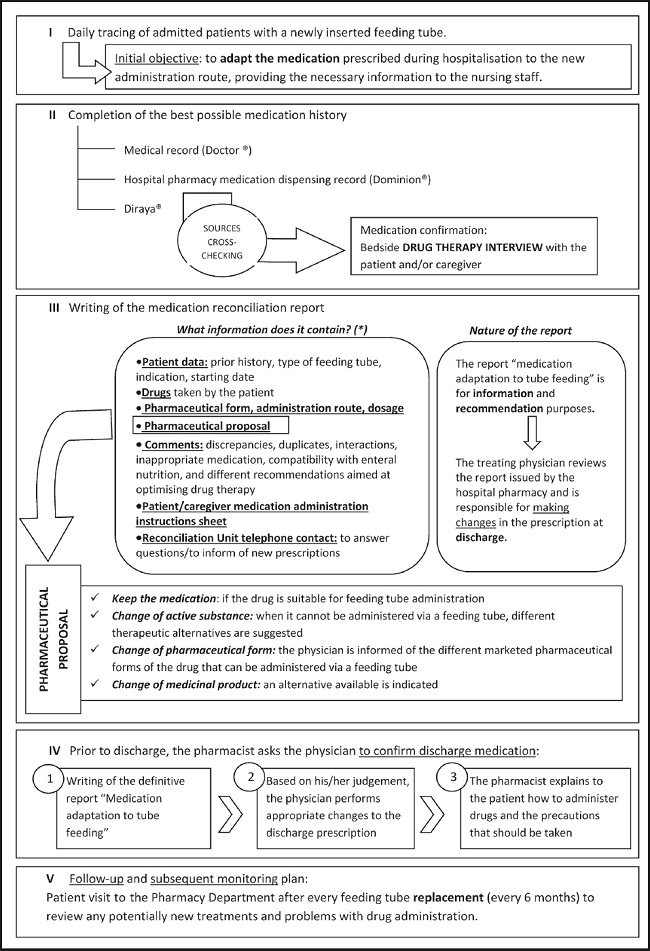
A multidisciplinary protocol for treatment adaptation for FT administration at discharge was implemented (Fig. 1). As shown, the pharmacists issued a discharge medication report with recommendations for the prescribing physicians. Additionally, they explained the changes to the patients/caregivers at a pharmaceutical care visit.
All the recommendations issued regarding drug administration via FT were based on published literature (5,6). When no published recommendations for FT administration were available for a specific drug, recommendations were based on the pharmaceutical form and absorption site of the drug, and the prescriber was informed about the lack of published information. A pharmacist report was issued at the first visit and all the follow-up visits.
Demographics (Table I), number of pharmaceutical care visits (first visit and follow-up visits), number of patients requiring changes to at least one drug, and number of recommendations related to adaptation of the drug to the route of administration were recorded. Recommendations were classified as follows:
− Change of active substance required due to: tube obstruction problems, drug instability caused by crushing tablet coating, lack of drug compatibility or toxicity studies when manipulating the dosage form (5,6).
− Change of pharmaceutical form required due to: absorption alteration resulting from the modification of the release characteristics of extended-release pharmaceutical forms, prioritisation of liquid forms, lack of drug compatibility or toxicity studies when manipulating the dosage form (5,15).
Table I. General characteristics of the study population.
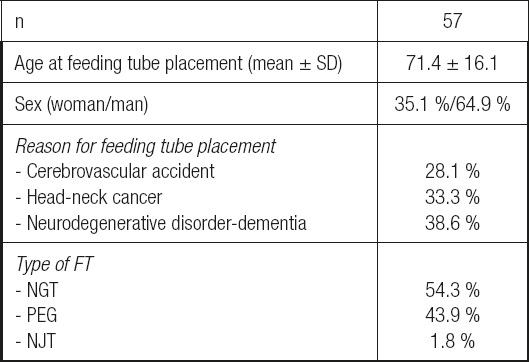
FT: feeding tube; NGT: nasogastric tube; NJT: nasojejunal tube; PEG: percutaneous endoscopic gastrostomy; SD: standard deviation.
Subsequently, acceptance of the recommendations by the prescribing physicians was recorded.
The statistical analysis was performed using absolute frequencies (percentages) for qualitative variables and central measures (mean) with dispersion measures (SD, range) for quantitative variables.
The study was conducted pursuant to the Spanish Organic Law 3/2018, of 5 December, on Personal Data Protection and Guarantee of Digital Rights and the Declaration of Helsinki. Patient privacy and confidential processing of the personal data were ensured.
RESULTS
A total of 57 patients were included in the study. Demographics, FT type, and reasons for FT placement are shown in table I.
A total of 66 pharmaceutical care visits were recorded, amounting to 1.2 visits per patient (nine were follow-up visits). Following treatment review, in 47 of these 66 visits (71.2 %), at least one drug modification was required in the patient’s prescription. The median number of drugs to be modified per patient was 2 (Interquartile Range [IQR], 1-3).
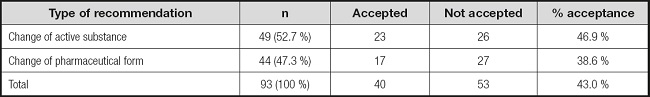
In the 66 visits performed, of the 489 drugs reviewed, a total of 93 had to be modified for FT administration (19.0 %). The primary change required was switching to a different active substance, representing 52.7 % (49/93) of the cases, followed by changes of pharmaceutical form in 47.3 % (44/93) of the cases (Table II).
The level of acceptance of the recommendations by physicians was 43.0 %. Acceptance by type of change suggested is shown in table II.
The recommendations to adapt a drug for FT administration that were not accepted by physicians were analysed. A change of active substance was recommended when no other medicinal product or pharmaceutical form compatible with FT administration was available. Twenty-six (53.1 %) of these recommendations from a total of 49 were not accepted. They were based on: FT obstruction problems caused by the drug (omeprazole, 11 prescriptions); drug instability resulting from crushing coated tablets (pantoprazole, 2 prescriptions; tamsulosin, 7 prescriptions); lack of compatibility studies (5 prescriptions); or drug toxicity following dosage form manipulation (dutasteride, 1 prescription).
For the recommendations to change the pharmaceutical form, 27 (61.4 %) of a total of 44 were not accepted. These recommendations were issued based on: alteration of drug absorption; changes to the drug release characteristics for extended-release pharmaceutical forms or forms with enteric coating (14 prescriptions); availability of a more suitable pharmaceutical form for tube administration requiring less manipulation (7 prescriptions); potential toxicity for the person manipulating the drug resulting from aerosols formed when crushing tablets (coated valproic acid tablets, 3 prescriptions); or lack of compatibility studies (3 prescriptions).
DISCUSSION
Our study reveals that approximately three in four patients receiving a FT for the first time require at least one change in their treatment at discharge because of incompatibility issues of the prescribed drugs with the administration route. This is in line with the findings of a study by Van Dem Bemt et al., in which errors were identified in 76 % of medication administrations during hospitalization in patients with FT (1).
Our findings reveal that every patient with recommendations to adapt a drug for FT administration had been prescribed a median of two drugs requiring changes, a figure slightly higher compared to other studies where the median number of drugs inappropriately administered through an FT was 1.34 (16).
Overall, 19 % of all the drugs studied had to be modified. This figure is similar to the one found in a study performed in an Intensive Care Unit (ICU) where 20 % of the drugs prescribed were found to be inappropriate for FT administration (10).
The recommendations issued were classified in two types (Table II). The most common one was switching to a different active substance. For example, omeprazole can cause mechanical problems leading to tube obstruction because it has to be administered as whole microgranules, which cannot be crushed as this could result in changes in drug bioavailability (5,17). Given the greater complexities of omeprazole administration compared to other proton pump inhibitors (PPIs), we recommended replacing it with other pharmacotherapeutically equivalent active substances that did not present the same problems, such as esomeprazole. In the case of pantoprazole, this drug is only marketed in a gastro-resistant oral tablet form and therefore cannot be crushed because of the instability of the active substance in an acid environment (18). Another drug that had to be changed was tamsulosin, which is exclusively marketed as extended-release tablets or capsules. Crushing the tablets or opening the capsules would have an impact on the drug’s pharmacokinetic characteristics (18). Recommendations were also given for cytotoxic drugs such as dutasteride because it can irritate the oropharyngeal mucosa when it is released from soft capsules and it can also cause foetal harm in pregnant women handling the substance as it is absorbed through the skin (5).
The second type of recommendation issued was changing a drug’s pharmaceutical form. One of the most common reasons for this type of adaptation was the availability of a more appropriate pharmaceutical form for FT administration. It is important to note that the bioavailability of coated tablets is altered when crushed. This was the case with the proprietary medicinal product Adiro® (acetylsalicylic acid) which has a gastro-resistant coating allowing the drug not to be released immediately in the stomach but in the duodenum in a delayed fashion (19). This type of coating can cause obstruction problems in FTs and therefore it is advisable to replace it with tablets that do not have an enteric coating (5,20).
Less than half of the pharmacists’ recommendations to switch to another active substance or to make changes to the pharmaceutical form were accepted by the physicians. This finding is in contrast to results from other studies showing a much higher degree of acceptance (between 82 % and 98 %) (21,22), although these studies focused on general medication reconciliation (MR) at discharge and not specifically on adaptation of the medication to FT administration.
The non-acceptance of recommendations by the prescribing physicians could stem from a lack of training on the compatibility of certain drugs with FT administration, leading them not to trust the recommendations issued. It may also result from a lack of knowledge of the different pharmaceutical forms available and the pharmaceutical characteristics of each one of them. Another potential factor is the lack of patient follow-up after discharge to Primary Care where medication adaptation to FT administration needs to be performed by primary care physicians, but the lack of contact with hospital pharmacists makes it a difficult process.
The low levels of physicians’ acceptance should be considered internally as a lack of understanding of the recommendations and show that the in-hospital pathway and prescribers’ training need improving.
The single-centre nature of the study and its small sample size resulting in a limited statistical power were the main study limitations. Moreover, research on medication adaptation for FT administration is scarce and further studies are necessary to draw more definitive conclusions.
We would like to conclude by highlighting that this study emphasises the importance of adapting medication to tube feeding and confirms that the inclusion of clinical pharmacists in multidisciplinary teams leads to improvements in patient drug therapy, as shown in multiple MR studies (21,23). In fact, specialised pharmacists have been shown to be more efficient in the reconciliation process than other healthcare professionals (24).