INTRODUCTION
Chronic kidney disease (CKD) is characterized by a total or partial and irreversible loss of renal function and is accompanied by endocrine, gastrointestinal, neurological, electrolytic and water alterations (1,2).
Fluid overload is important to evaluate due to its close relationship with cardiovascular complications, which are the main cause of death in renal patients regardless of age, dialysis modality, cause of end-stage renal disease (ESRD), race or geographic region (3-5).
In CKD, water overload is relevant in terms of its association not only with mortality but with nutritional alterations and systemic inflammation accompanied by an increase in catabolism, which further increases morbidity and mortality. This entity is known as protein-energy wasting (PEW) and has a prevalence of between 28 and 54 % in patients receiving replacement therapy (6,7); it is somewhat less common (11 to 54 %) in patients with stages 3 and 4 CKD (8,9).
PEW is also associated with an increase in the number of hospitalizations, longer hospital stays and higher morbidity and mortality (10,11). Therefore, an adequate evaluation of the nutritional status that includes body composition measurements is of vital importance (12).
At present, bioelectrical impedance analysis (BIA) is one of the most widely used methods for determining body composition because of its accuracy and ease use. BIA offers a noninvasive evaluation of the composition of the human body that allows estimation of total body water (TBW); furthermore, based on the physiological principle of constant tissue hydration, which assumes that 73 % of the fat-free mass (FFM) is water, BIA can obtain the FFM and consequently, the fat mass (FM), using a simple equation based on two compartments (FFM kg = total weight kg - FM kg). BIA analysis is based on the relationship between the electrical properties of the human body, the body composition of different tissues, and the total water content of the body (13). Like all indirect methods for estimating body composition, BIA depends on some assumptions related to body’s electrical properties, composition, state of maturation, hydration, age, sex, race and physical condition (13).
Given that in the vast majority of patients with kidney disease, the assumption of normality of hydration is not met, bioelectrical impedance vector analysis (BIVA) has been used to avoid biasing the diagnosis of body composition. BIVA is based on the components of bioimpedance (Z), resistance (R) and reactance (Xc), standardized by height and phase angle (PA), and not on estimates from prediction equations (14,15). This method has been well studied in patients in advanced stages of CKD who are receiving renal replacement therapy; however, its use has been less studied in patients in intermediate stages of CKD, which limits the possibility of testing its usefulness for detecting early changes in body composition that indicate fluid alterations and could help reduce the number of complications and mortality in this population. Therefore, our objective was to determine the association between the hydration status measured by BIVA and biochemical and clinical parameters and mortality in patients with stage G3a, G3b and G4 CKD.
MATERIALS AND METHODS
This is a subanalysis of data collected in two studies; the first one was a cross-sectional study of 81 patients recruited from the Salvador Zubirán National Institute of Medical Sciences and Nutrition (INCMNSZ) in 2004. The objective of this previous study was to evaluate hydration status using BIVA and to identify its association with anthropometric, clinical, and biochemical variables. The second study was a blinded randomized controlled trial of 57 participants recruited from the INCMNSZ in 2015, who underwent different clinical and nutritional assessments, including BIA.
For both studies, the inclusion criteria were as follows: patients from the nephrology outpatient clinic of the INCMNSZ with an estimated glomerular filtration rate (eGFR) of 15 to 59 ml/min/1.73 m2 (KDIGO stages G3a, G3b and G4) (16), and were males or females between 18 and 65 years of age. Exclusion criteria included patients who were receiving some type of replacement therapy; those who had amputations, pacemakers or a metal prosthesis; and those who had an erroneous BIA measurement.
Both studies were approved by the Ethics Committee and the Human Biomedical Research Committee of the INCMNSZ. All patients provided written informed consent to participate in the study.
CLINICAL AND BIOCHEMICAL ANTHROPOMETRIC DATA
A fasting blood sample was taken and was processed in the laboratory of the nephrology department to determine the following parameters: urea nitrogen, creatinine, glucose, sodium, potassium, calcium, phosphorus, albumin, cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, hemoglobin, hematocrit and albumin in urine. Systolic and diastolic blood pressure was measured by a lead nurse.
Body weight was measured in all patients with a Tanita BWB 800 scale (Tanita Corp., Tokyo, Japan), and height was measured with a Harpenden wall stadiometer (Holtain, Ltd., UK); these data were used to obtain the body mass index (BMI). The glomerular filtration rate was estimated with the CKD-EPI formula. All of this information, as well as the results of the BIA studies, was recorded on the data collection sheet for each participant.
BIOELECTRICAL IMPEDANCE ANALYSIS (BIA) DATA
For the BIA, the patients were asked to fast for a minimum of 4 hours prior to the study, not to perform strenuous exercise in the 24 hours before the study, not to consume alcoholic beverages in the 48 hours prior to the study, not to wear any metallic objects (e.g., jewelry) and, in the case of women, not to be menstruating. All patients were evaluated using a Quadscan 4000 multi-frequency device (Bodystat LDT®, Isle of Man, UK), which performed measurements at 5, 50, 100 and 200 kHz. For the BIA measurements, the patients were instructed to remove heavy clothing, glasses and shoes; to stay still; and not to speak during the measurement. BIA measurements were performed according to the criteria established by the National Institutes of Health Technology Assessment Conference Statement (17). The participants were placed in a supine position with their arms and legs away from the body and the palms of the hands facing down. Electrodes were placed in pairs on the right extremities, on the back of the hand and foot near the phalanx-metacarpal and phalanx-metatarsal joints, on the styloid process of the wrist and between the medial and lateral malleolus of the ankle for introducing the BIA current. After the measurement, the impedance value at a frequency of 50 kHz was collected from the equipment. This impedance value was used to determine the R, Xc and PA using the Bodystat Phase Software provided by the BIA analyzer manufacturer (Bodystat LDT®, Isle of Man, UK).
BIOELECTRICAL IMPEDANCE VECTOR ANALYSIS (BIVA)
BIVA does not depend on models, estimates or equations, since it is only affected by z-scores or individual variability. It is based on a graph called the RXc chart, which was created by Piccoli et al. (14) and in which the R standardized by height (R/H) is located on the abscissa and the Xc standardized by height (Xc/H) is located on the ordinate axis. Each individual vector can be compared with the reference population of ellipses that represent 50, 75 and 95 % by sex. The impedance vector can vary or can migrate to different areas; long vectors are interpreted as states of dehydration, short vectors are interpreted as overhydration, and migration to the left or right is considered indicative of changes in the body tissues (18,19). Different alterations in body composition can be detected by analyzing impedance vectors.
The R and Xc data standardized by height were plotted using the BIVA tolerance software and the reference values for Mexican population (20) to obtain the impedance vectors on the RXc graph. In order to conduct a group analysis according to the CKD stage and hydration status, the BIVA Confidence software was employed (21) following the methodology proposed by Piccoli et al. (22) , in which the values of R and Xc were transformed into z-scores (z [R] and z [Xc]) considering the reference intervals, which allows a set of tolerance ellipses independent of sex to be defined. Group vectors are represented as the mean and 95 % confidence intervals of the z-score.
MORTALITY
The electronic clinical record of each of the participants was reviewed to determine deaths from all causes as of March 2020. Only the date of mortality was recorded and not the cause.
STATISTICAL ANALYSIS
The general characteristics of the participants are presented as measures of central tendency and dispersion according to their distribution and the type of variable. Continuous variables are presented as mean and standard deviation or median and interquartile range. Categorical variables are presented as frequencies and percentages. To determine the distribution of the variables, the Kolmogorov-Smirnov normality test was used.
A Pearson (parametric variables) or Spearman (non-parametric variables) correlation analysis was performed to evaluate the relationship of the BIVA components (R, Xc, PA, R/H and Xc/H) and other clinical and biochemical indicators. The coefficients were interpreted as follows: 0-0.29 = weak correlation; 0.30-0.69 = moderate correlation; and 0.70-1.0 = strong correlation (23). The individual hydration status was assessed using the BIVA tolerance program (21). For group analysis, the differences between the group vector patterns were analyzed with the Hotelling t2 test. To compare the anthropometric, biochemical and clinical parameters according to hydration status and CKD stage, an independent samples t2 test was used for variables with parametric distribution, and the Mann-Whitney U analysis was used for variables with a nonparametric distribution. To compare the variables according to the CKD stage, one-way ANOVA was used for parametric variables, and the Kruskall Wallis test was used for nonparametric variables. Survival analysis was performed using the Kaplan-Meier method and weight adjusted Kaplan-Meier method (24) to make unbiased comparisons between time-varying groups and control for confounding (sex, age, and body mass index), and the log-rank test was used to identify differences between the curves according to hydration status.
We assessed the multivariate effects of covariates with the Cox proportional hazard analysis to estimate the relative hazards of the mortality crude and adjusted by the following base-line variables: model 1, age group, sex, body mass index, and model 2, age group, sex, body mass index and eGFR. Hazard ratios (HR) and corresponding 95 % confidence interval (CI) were estimated.
The SPSS 26 program was used to perform the statistical analyzes, and the MedCalc program was used to obtain the survival curves. A p-value < 0.05 was considered statistically significant.
RESULTS
Table I shows the general characteristics of the population, as well as the clinical and biochemical parameters and the impedance components. Most parameters were in normal ranges, with the exception of calcium concentrations 8.9 (8.4-9.4), triglycerides 187.9 ± 71.7, hematocrit 34.9 (31.7-40.8) and uremic toxins, such as urea nitrogen 44.6 (34.1-63.6) and creatinine 2.23 ± 0.8.
Table I. General characteristics, clinical and biochemical parameters, and bioelectrical impedance analysis components in the study population.
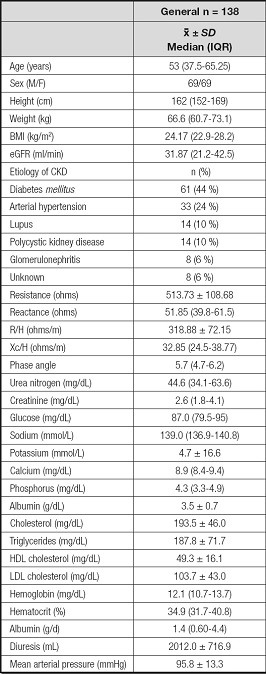
BMI: body mass index; eGFR: estimated glomerular filtration rate; R/H: resistance/height; Xc/H: reactance/height.
Positive associations were found between the three BIA components and albumin (albumin and R/H, r = -0.38, p = 0.001; Xc, r = 0.59, p ≤ 0.0010; PA, r = 0.58, p ≤ 0.0010). Other biochemical parameters that showed positive correlations were hemoglobin concentration and hematocrit, although they were correlated with only two of the BIA components, Xc/H and PA (hemoglobin and Xc/H, r = 0.31, p = 0.01; hemoglobin and PA, r = 0.44 p = 0.000); hematocrit and Xc/H, r = 0.30, p = 0.010; hematocrit and PA, r = 0.44, p ≤ 0.0010). In contrast, a negative correlation was observed between blood glucose concentrations and Xc/H and PA (glucose and Xc/H, r = - 0.41, p = 0.025; glucose and PA, r = -0.44, p = 0.015).
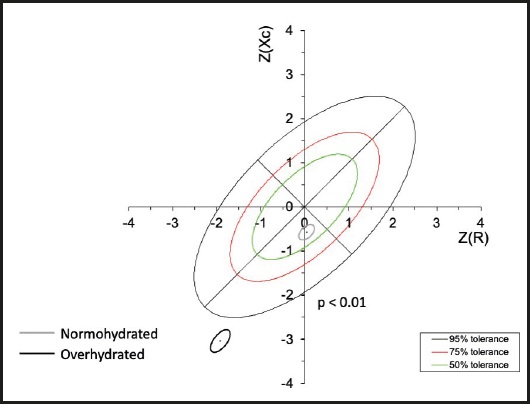
Subsequently, the R/H and Xc/H values were plotted on the impedance vectors of the reference population. Patients with overhydration and normal hydration were identified and were plotted as groups on the z-score graphs (Fig. 1).
Table II shows the differences in the evaluated parameters between the normally hydrated and overhydrated patients according to the BIVA. There was higher proportion of women (61 %) among the patients with volume overload; additionally, the patients in this group were older and heavier and showed lower values of R, Xc and PA values than the patients with normal hydration. The patients with volume overload also had lower concentrations of albumin, calcium, hemoglobin and hematocrit, greater albuminuria and a higher mean arterial pressure.
Table II. Anthropometric, bioelectrical impedance, clinical and biochemical parameters of the overhydrated population compared to normohydrated patients.
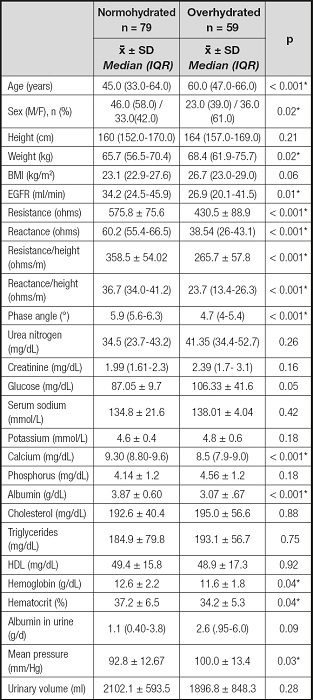
eGFR: estimated glomerular filtration rate. Differences between normohydrated and overhydrated patients: *p < 0.05.
Non-parametric analyses were used in variables reported by median and interquartile range (IQR). Parametric analyses were used in variables reported by mean and standard deviation.
Table III shows the differences in the evaluated parameters by CKD stage. Statistically significant differences in the proportions of patients with overhydration were found, with a greater prevalence among patients in stage G3b (53 %). PA also showed significant differences between the stages of CKD; it was smaller in the patients in more advanced stages. Regarding the biochemical parameters, differences were observed in the uremic toxins BUN and creatinine,in addition to phosphorus concentrations, hemoglobin, hematocrit (and in urinary volume).
Table III. Anthropometric, electrical bioimpedance, clinical and biochemical parameters of the study population according to stage of chronic kidney disease.
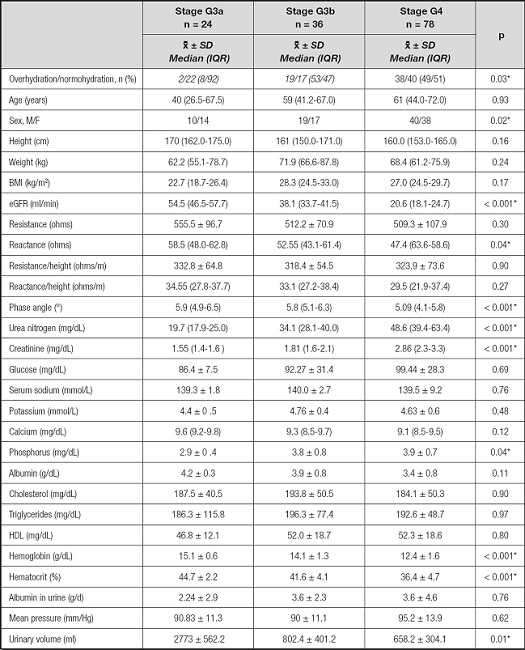
Diffrences between patients by stage of chronic kidney disease, *p < 0.05.
Non-parametric analyses were used in variables reported by median and interquartile range (IQR). Parametric analyses were used in variables reported by mean and standard deviation.
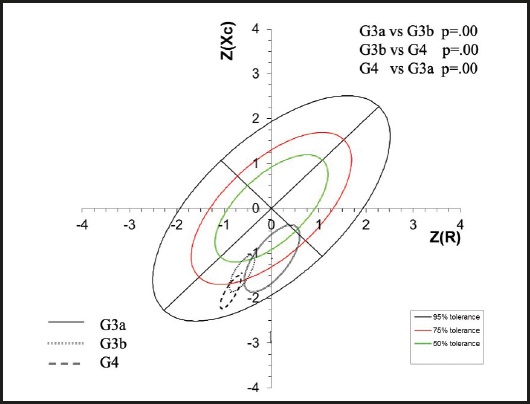
Subsequently, the means of the vectors were plotted by group according to the z-scores for CKD stage. Three groups were located in the lower right quadrant, which indicated a pattern of both overhydration and cachexia. However, for the patients in stages G3a and G3b a portion of the sample fell within the normal quadrants, while the majority of the patients in stage G4 were located below the tolerance ellipse of 75 %; in other words, migration of the mean of the vector towards the lower part of the ellipses is noted as the CKD advances (Fig. 2).
The mean duration of follow-up was 119.6 months (median, 65.5 months). Of the 138 patients included in the mortality prediction analysis, 12 (8.7 %) died, with a median of 52.2 months (IQR: 38.5-77.7 months) of follow-up until death was recorded. Nine (75 %) patients who died were in the overhydrated group. Patients in the overhydration group were significantly more likely to die, as demonstrated by the Kaplan-Meier curves (Fig. 3).

Figure 3. Survival of normally hydrated and overhydrated patients according to impedance vectors adjusted by aged sex and body mass index.
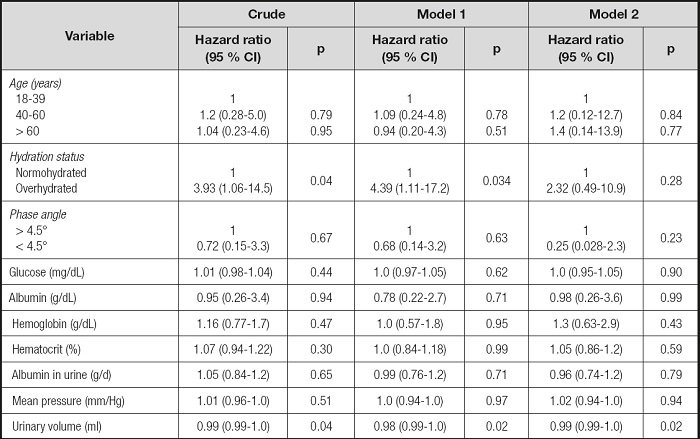
In table IV, multivariate Cox proportional hazards regression showed that increased mortality risk was associated (p < 0.05) with hydration status and urine volume except for adjusted hydration status by age, sex, BMI, and eGFR. Patients with overhydration status had a 4-fold greater risk than normohydrated patients for mortality (HR, 4.39; 95 % CI, 1.11-17.2). Higher urinary volume was associated with a lower risk of mortality (all p < 0.05). The rest of the variables did not show a statistically significant association with mortality.
DISCUSSION
The present study included patients in the intermediate stages of CKD who were not receiving replacement treatment, because at this stage, some patients show the onset of complications, including alterations in body composition. The purpose of the study was to analyze body composition through the BIVA method, with the objective of demonstrating the clinical utility of this tool for improving medical and nutritional treatment, even before any type of dialysis begins.
Regarding the BIA components, low average PA, R/H and Xc/H values were observed for the entire population. This allowed the estimation of short vectors that indicate water retention (14,25), a clinical sign that is present primarily in the advanced stages of CKD but is not necessarily absent in the intermediate stages, as could be observed in the BIVA graphs. This highlights the relevance of BIVA at any stage of CKD; using the results of conventional BIA can lead to bias because these values use prediction equations that are based on healthy populations without alterations in body components.
Regarding biochemical parameters, the general population showed normal concentrations, with the exceptions of serum concentrations of urea nitrogen and creatinine, which is to be expected in cases of renal disease progression (26). An increase in triglycerides and decreases in calcium, hemoglobin and hematocrit were also expected to be observed.
The patients showed normal diuresis and mean arterial pressure, while their median glomerular filtration rate was 31.87 (21.2-42.5) ml/min, which can identify the general stage G3b population, according to the KDIGO classification (27). This represents a decrease in the glomerular filtration rate from moderate to severe, a situation associated with the following conditions, which are listed from the most to the least prevalent according to the Spanish Society of Nephrology (27): hypertension, hyperparathyroidism, anemia, acidosis, vitamin D deficiency, hyperphosphatemia and hypoalbuminemia. The study population presented a decrease in calcium concentrations, most likely associated with vitamin D deficiency, as well as anemia; however, participants did not present a lack of blood pressure control, even though this is the second most common cause of CKD, nor were alterations in blood concentrations of phosphorus and albumin observed. It is worth mentioning that the presence of acidosis was not evaluated.
When groups of patients were graphed onto the z-score ellipses (15), it was observed that a significant percentage of the population had alterations in water status (43 %), and these patients showed a higher glomerular filtration rate (Table II). Some studies (28,29) have described that as a patient progresses to CKD, alterations in body composition occur. It has been shown that FM and lean mass decrease significantly as the effects of anorexia and low nutritional intake begin to emerge, generating a common catabolic state called PEW syndrome. These alterations in body composition can be partially stabilized with effective treatments to control uremia and with an increased dietary intake. However, a complete normalization of FM and lean mass rarely occurs (28).
Patients who were identified as overhydrated were older, heavier and, as expected, had lower R, Xc and PA values than patients without volume overload. This finding has been mentioned in other studies (25), since it has been established that with BIVA, an individual’s hydration level is determined by vector length in such a way that short vectors indicate greater body fluid volumes and less resistance, while a lower PA is associated with deterioration of nutritional status and an increased risk of mortality (20,25,30). Xc, on the other hand, determines the capacity of cell membranes to store energy, since they act as electrical capacitors; when an electric current passes through them, they act as conductors, and the cellular content acts as a dielectric material, storing the charge when the current passes between the intracellular and extracellular compartments. In this way, when there is alteration in the cell membranes, as in the case of fluid retention, the Xc decreases.
Regarding the biochemical parameters of the overhydrated patients (Table III), lower blood concentrations of albumin, hemoglobin and hematocrit and higher albuminuria and mean arterial pressure were observed. These results could have occurred for two reasons: first, and with a focus on albumin concentrations, hypoalbuminemia is an important risk factor that increases both mortality and morbidity in patients with CKD; however, it must be considered that although hypoalbuminemia is normally attributable to malnutrition or wasting or a chronic inflammatory state, it can also be the result of hemodilution caused by volume expansion with respect to the low concentrations of hemoglobin and hematocrit, and it can be explained by the secondary presence of anemia in renal disease. It is worth mentioning that both groups of patients showed very low values for these two variables and therefore, we cannot refer to hemodilution alone as the main cause of these low concentrations. Albuminuria was more common in patients with overhydration, as expected; this finding is related to hypoalbuminemia and decreased oncotic pressure. The presence of volume overload can also have repercussions for blood pressure; in the patients with overhydration, the mean arterial pressure was significantly higher in the group with fluid retention, although it is worth mentioning that within normal ranges.
Regarding the correlations between the BIA components (R/H, Xc/H and PA) and the biochemical and clinical parameters, a significant association was found between the BIA components and albumin. This association can corroborate the diagnosis of overhydration by BIVA, since albumin, hematocrit and hemoglobin are closely related to extracellular fluid content. The correlations found in the present study are similar to those identified by Picolli et al. (31) in 1998, in which albumin and hemoglobin were correlated in a group of hemodialysis patients; this study found the albumin concentrations were negatively correlated with R/H and positively correlated with Xc/H, while hemoglobin was positively correlated with both BIA components. It is worth mentioning that the correlation coefficient obtained for most of the evaluated variables was lower than r = 0.6, indicating moderate correlations.
The negative correlation between blood glucose concentrations and Xc/H is noteworthy because there are no studies that have directly observed this relationship. The only related study is one by Amaral et al. conducted in 2007 (32), which investigated alterations in glucose concentrations in aqueous solutions and blood samples using spectroscopic impedance and found that R and PA were the most informative parameters. These parameters reflect changes in the capacity of the cell membrane and therefore show that the number of hematocytes or the volume increase proportionally with glucose levels.
In the analysis by CKD stage, as expected, significant differences were found in eGFR, BUN, creatinine, phosphorus, hemoglobin, hematocrit and urinary volume, due to the progression of the disease itself and as it progresses, changes in composition. body and biochemical parameters are more present (33).
The most frequent cause of mortality in advanced CKD is cardiovascular disease (CV). Some known CV factors (traditional and new), such as male sex, age and diabetes, are associated with overhydration and have an increased prevalence in overhydrated patients (34-36). Although this study did not found in the multivariate Cox regression analysis other causes that influence the mortality of patients with CKD in intermediate stages (probably due to small sample size), one of its findings was that overhydration itself is a long-term risk factor for mortality. In fact, our results coincide with those of studies by Vega et al. (37) and Bansal et al. (38) in which a higher mortality rate was found in patients with short vector lengths, which denote greater overhydration in patients in the intermediate and advanced stages of CKD. Therefore, BIVA is an easy and useful tool that can be used to help detect high-risk patients (39). Although our study lacks longitudinal measurements, it is likely that the frequently use of BIVA, for example, at each clinical visit, could improve patient survival (40), since patients with overhydration could receive closer follow-up, adjustments of diuretics use if necessary, and more frequent visits to the nephrologist.
Body composition influences mortality in the intermediate stages of CKD, as previously described (41,42). Therefore, it is important to monitor body composition before the final stages of kidney disease. BIVA is a useful tool that can be correlates with clinical and biochemical parameters to evaluate alterations in the hydration status of patients in stages 3 and 4 of CKD.
We recognize two main limitations of our study. One is the failure to study the association between mortality and other factors (such as cause of mortality, comorbidities, inflammation, medication, etc) in addition to overhydration. The other is the probable underreporting of deaths in the clinical record; if the death did not occur in the hospital, it is not registered, and therefore, the mortality rate could be lower and comparable to that reported in other studies (37).
CONCLUSIONS
Patients who are diagnosed with fluid overload by BIVA have lower concentrations of calcium, albumin and hematocrit; lower PA values; and higher mean arterial blood pressure values than patients with normal hydration, indicating an association between these variables and hydration status. Patients with overhydration according to BIVA have a higher mortality compared to those who do not present overhydration. BIVA is a useful tool for the evaluation of body composition and hydration status in patients with stage G3 and G4 CKD who are not undergoing dialysis.