INTRODUCTION
Sodium is an essential chemical element for organic homeostasis, acting on plasma volume, acid/basic balance, synaptic transmission and cell metabolism (1). It is the most abundant ion in the extracellular medium, added to the two main anions, chlorine and bicarbonate, form 90 % of the extracellular solute (2).
Excessive consumption of sodium chloride is related to changes in blood pressure, volume overload and proteinuria, negatively influencing the onset and development of chronic kidney disease (CKD) (3,4).
Reducing sodium in the diet by 30 % is one of nine targets approved by the World Health Organization Assembly to reduce the overall burden of non-communicable diseases by 25 % by 2025 (5).
Considering that approximately 93 % of the sodium consumed is excreted in the urine of 24 h, thus 24-hour urinary sodium can be used to provide an approximate estimate of dietary intake in population studies (6).
This study aimed to evaluate the correlation between sodium intake estimated by dietary intake and 24-hour urinary excretion in CKD patients.
MATERIAL AND METHODS
This is an analytical, cross-sectional study conducted at the Center for the Prevention of Diseases (CPDR) of the University Hospital of the Federal University of Maranhão (HUUFMA).
The study included individuals with CKD in stages 2, 3A, 3B and 4 (GFR 15-89 mL/min/1.73 m²), aged 18 years or older, followed at the outpatient clinics of the CPDR, a unit that is part of HUUFMA, which is a reference in the State for the diagnosis and treatment of neonatal diseases. Patients with chronic consumptive diseases, pregnant women, urinary infection, autoimmune diseases and use of loop diuretics were not included.
The sample size was calculated considering the mean sodium excretion estimate of 154.1 ± 69.6 mEq/day, (7), significance level of 5 % and power of 90 % to detect a difference of at least 0.3 mEq/day. Thus, the minimum size required was 119. Considering possible losses (25 %), totaling 149 patients.
Data were collected from October 2018 to July 2021 at CPDR. The selected individuals were informed of the object of the study and, those who agreed to participate, signed the free Informed Consent Form (ICF). They then answered a questionnaire regarding socioeconomic, demographic, clinical and lifestyle information. They were also instructed about the process of collection of laboratory tests and anthropometric evaluation.
The socioeconomic and demographic variables of interest were: gender, age (in years) skin color (self-reported), family income (minimum wages), schooling (in years of study). Clinical and lifestyle variables, staging of CKD (stages 2, 3A, 3B and 4), diagnosis of diabetes mellitus (DM) and systemic arterial hypertension (SAH), physical activity, smoking and alcohol consumption were also evaluated.
Blood pressure (BP) measurement followed the recommendations of the Brazilian Guidelines on Arterial Hypertension, 2020 (8). An automatic oscillometric sphygmomanometer duly validated by the British Hypertension Society and Association for Advancement of Medical Instrumentation was used (9). Three measurements were performed, with an interval of 1 to 2 minutes and additional measurements only if the first two readings differed > 10 mmHg. The average of the last two was recorded. BP was considered high when systolic BP (SBP) was ≥ 140 mmHg and/or diastolic BP (DBP) ≥ 90 mmHg (8).
Anthropometric evaluation was performed by means of body weight, height and waist circumference (WC) measurements. Bo- dy weight (in kg) was measured with the aid of a calibrated scale (Filizola®, Brazil) with a maximum capacity of 150 kg and subdivisions per 100 g.
Height (in cm) was obtained with the aid of a portable statometer (Alturexata®, Brazil) with a scale from 0 to 220 cm and precision of 0.1 cm. To evaluate the adequacy of weight for height, the body mass index (BMI) was calculated, obtained by the ratio between weight and height square. For the evaluation of central adiposity and cardiovascular risk, WC was measured at the midpoint between the last rib and the iliac crest at the time of expiration, using non-extensible anthropometric tape measure (Sanny®, Brazil).
Blood samples were collected after 12-hour night fasting and included fasting glucose (GJ) (colorimetric method), creatinine (Cr) (Jaffé reaction), total cholesterol (TC) (colorimetric method), HDL-cholesterol (automated molybderate UV method), LDL-cholesterol (electrochemiluminescence method), triglycerides (TG) (chemiluminescence method), parathyroid hormone (PTH), and 25OH vitamin D (chemiluminescence method).
To define CKD, two previous evaluations of renal function were considered, with a minimum interval of 3 months, according to the orientation of the KDIGO (10). Renal function was evaluated using the estimated glomerular filtration rate (GeTF), obtained from the CKD-EPI equation (11) that uses serum creatinine as a function marker. Renal function reduced by a GFR below 60 mL/min/1.73 m² was considered reduced renal function. For the staging of CKD among the patients included in the study, the classification of stages was considered: 2 (GeTF 60-89 mL/min/ 1.73 m²), 3A (GeTF 45-59 mL/min/1.73 m²), 3B (GeTF 30- 44 mL/min/1.73 m²) and 4 (GeTF 15-29 mL/min/1.73 m²) (10).
Sodium excretion was evaluated in 24-hour urine. Patients were carefully instructed to pack urine in appropriate vials (bottles of mineral water), discard the first urine of the initial day of collection, and from there collect all urine produced during the 24-hour period and keep it under refrigeration. The samples were considered accurate if the total urine volume was greater than or equal to 500 ml or with urinary creatinine > 15 mL/kg/24 h (men) and > 10 mL/kg/24 h (women).
Food intake was evaluated by applying three 24-hour food records (R24h) and individuals reported all food and beverages consumed, schedule, quantities in units of homemade measures, preparation, as well as place of consumption (inside or outside the home) on the day before the interview. The R24h was applied by a trained nutritionist using a “photo album” with photos of food portions and utensils from a photographic registry book for dietary surveys (12). Next, quality control of the information collected through the quantification of food and beverages was carried out in a standardized manner, with the help of the Table for Evaluation of Food Consumption in Homemade Measures (13). Finally, the data were entered in the Excel software and the sodium estimate of the mentioned foods were taken from the Brazilian Table of Food Composition (TACO) and from the United States Department of Agriculture (USDA) table, as well as consultations with food labels.
The statistical analysis was performed in the Stata® version 14.0 program, considering the significance level of 5 %. Categorical variables were presented by means of frequencies and percentages, and numerical variables, by mean and standard deviation (± SD) or median and interquartile interval (p25-p75). The normality of the numerical variables was verified by the Shapiro-Wilk test.
To compare the demographic, anthropometric, nutritional and laboratory variables with the tercis of sodium excretion in 24-hour urine of the participants, variance analysis (ANOVA) or Kruskal-Wallis was used.
Spearman's correlation coefficient was used to evaluate the correlation between 24-hour urinary sodium excretion and dietary sodium intake using R24h.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/ or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The research protocol was approved by the Research Ethics Committee of the University Hospital of Federal University of Maranhão (consolidated opinion No. 2.904,987), in compliance with the requirements of the Resolution of the National Health Council No. 466/12 and its supplementary norms for research involving human beings.
RESULTS
A total of 151 individuals were evaluated, their mean age was 60.8 ± 11.8 years and 51.7 % of these individuals were female. There was a higher frequency of individuals with complete elementary education (47.3 %), with family income lower than two minimum wages (77.3 %), and self-reported being brown (60.4 %). They reported: smoking, 30.2 %; alcohol consumption, 34.0 %; and physical activity, 52.7 %. Hypertensive patients were 88.9 % and diabetics 45.0 %. Most participants were in stage 3B of CKD (39.1 %) (Table I).
Table I. Demographic, socioeconomic, lifestyle and clinical characterization of patients with CKD on non-dialysis treatment, São Luís, Maranhão, Brazil, 2021.

MW: minimum wage; SAH: systemic arterial hypertension; DM: diabetes mellitus; CKD: chronic kidney disease.
When evaluating the association of sociodemographic, nutritional, clinical and laboratory variables with sodium excretion in tertiles, only HDL-c and PTH presented statistical significance. The individuals belonging to the highest tertile of sodium excretion (T3) presented lower PTH values when compared to tertile (T1 and T2) (p-value = 0.049) and those belonging to the lowest tertile (T1), presented higher serum HDL-c levels in relation to the highest tertile (T2 and T3) (p-value = 0.033) (Table II).
Table II. Distribution of sodium excretion tertiles with sociodemographic, nutritional, clinical and laboratory variables of chronic kidney disease patients, São Luís, Maranhão, Brazil, 2021.
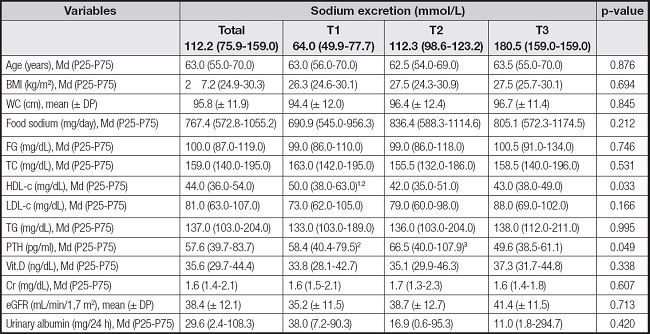
T: tertile; WC: waist circumference; FG: fasting glucose; TC: total cholesterol; HDL-c: High density lipoprotein-cholesterol; LDL-c: low density Lipoprotein-cholesterol; TG: triglycerides; PTH: parathyroid hormone; Vit D: vitamin D; Cr: creatinine; eGFR: estimated glomerular filtration rate.
1Tertile 1 vs tertile 2;
2tertile 1 vs tertile 3;
3tertile 2 vs tertile 3;
p-value < 0,05.
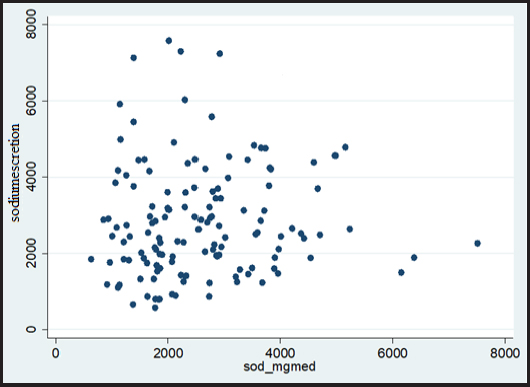
The median and interquartile interval of sodium excretion in 24-hour urine was 112.2 (75.9-159.0) mmol/L and the sodium intake evaluated by R24h, 833.8 (632.0-1028.3) mg/day. There was no statistically significant correlation (p-value > 0.241) of sodium as evaluated through dietary intake and excretion in 24-hour urine (Fig. 1).
DISCUSSION
In the present study with patients with non-dialysis CKD, the median sodium excretion in 24-hour urine was 112.2 mmol/L and sodium intake by R24h, 833.8 mg/day. There was no statistical correlation between dietary sodium intake and 24-hour urine excretion. The individuals belonging to the highest tertile of sodium excretion (T3) presented lower PTH values and those belonging to the lowest tertile (T1) presented higher serum HDL-c levels.
Sodium intake in this population compared to other studies was low; however, some points that may be related to this low intake should be highlighted. These individuals have CKD in multidisciplinary follow-up regularly in a reference center and already routinely receive guidance regarding the need for control in sodium intake, another point is that the intake may have been underestimated taking into account that only the intrinsic sodium of the food was evaluated, not taking into account the addition of sodium.
A study conducted with a population of ethnic characteristics similar to this population identified a mean sodium excretion well above 203.1 ± 84.9 mmol/day (14). Diet is a modifiable factor in the progression of CKD and high salt intake is associated with increased risk of progression (15).
An analysis of four prospective studies in CKD identified in a population > 65 years of age a median 24-hour sodium excretion of 143 mEq [interquartile range (IQR), 109-182] (16). Sodium intake has negative consequences on CKD. In dialysis patients, mortality, proteinuria and blood pressure increase. In non-dialysis, it also induces the worst blood pressure control, edema and cardiac alterations, despite improving nutritional status by appetite induced by salt intake and the amount of food, which is proportional to the amount of salt, which would be a beneficial factor, but does not overcome cardiovascular risks. Therefore, sodium intake is strongly related to extracellular fluid volume, blood pressure, appetite, nutritional status and mortality (17).
There was no correlation between 24-hour urinary sodium excretion with 24-hour R, which may demonstrate that R24h has limitations to determine the actual sodium intake in this population. Possible factors would be the use of diuretics that increase sodium excretion, such as thiazoids and loop, in addition to impaired renal management of sodium ion associated with loss of nephrons functioning with disease progression. A meta-analysis study revealed that R24h underestimated the average sodium intake of the population by 607 mg per day compared to 24-hour urine collection. However, R24h can be used if 24-hour urine is not feasible. Monitoring the sodium intake of the population with 24-hour urinary excretion remains the most accurate evaluation method (18). Thus, considering that more than 95 % of the quantity ingested of sodium is excreted in the urine, and that the dietary evaluation presents many biases, the urinary excretion of 24 hours constitutes an excellent marker of its daily consumption (19).
This study identified that individuals belonging to the highest tertile of sodium excretion (T3) presented higher serum HDL-c levels. Prospective cohort study conducted in Iran aimed at preventing chronic non-communicable diseases through the development of intervention programs that promote healthy lifestyles, revealed that individuals with dyslipidemia and high blood pressure in the highest quartile of the Dietary Approaches to Stop Hypertension (DASH) diet score had higher levels of HDL-c and lower TG levels at the beginning of the study and a higher level of HDL-c at the end of follow-up. In all subgroups, participants in the highest quartile of the DASH diet score with low sodium content had higher protein and carbohydrate intake. Confirming that a healthy eating pattern should be followed for prevention and delay in the progression of CKD (20).
The decrease in the number of nephrons accounts for numerous abnormalities that develop in the kidneys and the remaining nephrons allow the individual to survive. As GFR decreases, more sodium is excreted by the remaining nephrons. This indicates that a person with very low GFR < 5 ml/min ingesting 120 mEq/day of sodium excretes more than 30 % of the filtered sodium load to maintain balance (21).
It is known that CKD is characterized by several changes in mineral homeostasis. An experimental study conducted with nephrotomized dogs hypothesized that if phosphate intake is restricted from the beginning of nephron reduction, secondary hyperparathyroidism can be prevented. If phosphate intake is reduced in proportion to the decrease in GFR, there is no need for adaptation. The researchers identified that there were no changes in serum phosphorus, calcium, magnesium, tubular phosphate reabsorption or PTH, clearly indicating that the development of secondary hyperparathyroidism could be prevented, preventing the adaptation of phosphate excretion (22).
There was a higher number of hypertensive individuals, 88.9 %. Data from the 2020 Dialysis Census indicate SAH as the main cause of CKD followed by DM in Brazil (23).
Hypertension is one of the main contributors to the progressive loss of renal function and the development of cardiovascular disease in patients with CKD (24). A study conducted with 1,814 Australians with non-dialysis CKD showed that blood pressure control rates in hypertensive patients with CKD were far from ideal and people with CKD over 65 years of age and with albuminuria or proteinuria had a higher risk of progression of kidney disease and had higher rates of uncontrolled BP (25).
Our study has some limitations. The first is the study sample, the subjects are already followed, so it has a relatively lower sodium intake, as they are already oriented and evaluated periodically. Another limitation is that R24H is prone to recall bias, which can be systematic or random, and the estimation of sodium intake based on R24H excludes salt added to the table. Therefore, R24H should be used in conjunction with sodium intake assessment tools, such as 24-hour urinary excretion. As a strong point of this study, we highlight the use of a reference marker for sodium intake. This study shows that 24-hour urine collection remains the best method of dietary sodium intake for accurate measurement of sodium intake in the population.
CONCLUSION
The present study demonstrated that in patients with non-dialysis CKD waist circumference, HDL-c and PTH were the variables that were associated with urinary sodium excretion. There was no correlation between urinary sodium excretion in 24 h and food intake. The absence of correlation confirms the limitation of the evaluation of sodium intake via food intake, since the 24-hour dietary recall proved to be unreliable as a method of estimating sodium intake, as it usually underestimates or overestimates sodium intake. The evaluation of urinary sodium excretion is considered a reference method, besides, it is an excellent marker of its daily consumption and indicates the need for educational actions aimed at optimizing long-term salt restriction adherence.