INTRODUCTION
The brain is more sensitive to oxidative stress than other organs. It has low defensive antioxidant potential, high oxygen consumption, low oxidation resistance of neurotransmitters and their precursors, and lipid constitution of the neuronal membrane with unsaturated fatty acids that are very susceptible to lipid peroxidation to the active process of immune defense. These factors can trigger an excitotoxic process with the free radical generation, edema formation, and activation of cellular apoptosis (1-3).
Oxidative stress and inflammatory processes can negatively affect cognitive functions. The cumulative effect can potentially impact deterioration, especially of executive functions; disability manifests itself mainly in senescence. The term “executive function” corresponds to skills, including planning, organization, problem solving, self-monitoring, and decision making. These skills require integrating information from various cognitive domains to perform more complex tasks. These functions imply several skills, such as attention, concentration, abstraction, behavior initiation, cognitive flexibility, inhibiting control, working memory, and selectivity of stimuli (2,4,5).
Working memory is fundamental for the execution of several tasks involving executive functions, such as self-regulation, inhibition, and cognitive flexibility, influencing various behaviors. One of these behaviors is the decision about food intake. Such skills, when recruited in an integrated way, can restrict the properties that encourage inadequate eating cues resulting from impulsivity, remaining interdependent with cortical excitability, that is, the favorable situation to the inhibitory pattern is reinforced at the time of hyperpolarization; on the other hand, the excitatory pathway occurs favorably in cases with continuous neuronal depolarization (2,3,6).
Experimental studies with rats demonstrated that manipulations that weakened the inhibition of working memory also reduced rodent self-control over food intake and increased the likelihood of overeating (6-9). Rodents with a deficit in the inhibition of working memory presented greater food impulsivity, weight gain and/or overweight; the inverse finding was also validated. When rodents had more significant memory inhibition, they showed less food impulsivity (6,7,10,11).
Neuroplasticity and cortical excitability changes may contribute to neural activity regulation. Both could be modified by applying direct electric current in the sensory-motor cortex, with results dependent on the type and modality of the polarity of the current. Its effect would last for days after the end of stimulation (12,13).
In the context of invasive stimulation, deep brain stimulation and vagus nerve stimulation, performed only surgically, are examples of this type of stimulation. Regarding noninvasive brain stimulation patterns, Repetitive Transcranial Magnetic Stimulation (rTMS) and transcranial direct current stimulation (tDCS) can be mentioned. Noninvasive methods are more attractive for humans because they have the best cost-benefit ratio and allow painless modulation of cortical activity and excitability, applied through the intact skull (13,14).
For didactic purposes, the cerebral hemispheres are delimited with points; the right hemisphere is marked with even numbering and the left hemisphere with odd total. The letters correspond to the cerebral lobes; therefore, in this logic used internationally by the 10-20 system in the electroencephalogram, the Right Dorsolateral Prefrontal Cortex (CPFDL) will be registered as F4. The left one will be recorded as F3, both cortices belonging to the most central frontal lobe region (letter F).
Among the described effects of using ETCC, the following stand out: increased reaction time and mental flexibility accuracy scores (problem solving or constantly changing situations) and working memory (phonological and visuospatial loops). And although the variability of the protocols used is extensive, anodic stimulation in the right dorsolateral prefrontal cortex seems to have better results (13,15-19).
Although there are many publications on food desire and assessment of nutritional status or executive functions, there is little information on the effect of brain stimulation on executive functions in overweight or obese individuals. It is known that metabolic syndrome and its related components (obesity and chronic non-communicable diseases such as hypertension and type 2 diabetes mellitus) incarnate high long-term costs (20,21).
Considering the application of low-intensity electrical and/or magnetic current excitation modulation in cortical regions, the following question arises: How can noninvasive neuromodulation influence the performance of executive functions in overweight or obese individuals?
However, few studies simultaneously analyze the influence of noninvasive neuromodulation on the executive functions of overweight or obese individuals. Given the above, the research aimed to verify, through a literature review, the effects of neuromodulation on the performance of executive functions when applied to overweight or obese individuals.
METHODS
This is a systematic review article based on articles selected from the databases PubMed, ScienceDirect, BIREME (Latin American and Caribbean Center for Information in Health Sciences), and Web of Science. The search was performed using the following combination of descriptors: (“troubleshooting” OR “executive function” OR memory) AND (tDCS OR TMS) AND obesity.
Only controlled and/or randomized clinical trials published in any language were analyzed between 2011 and May 31, 2022, using the PICOS (Population, Intervention, Comparator, Outcome, and Study Design) strategy to assist in the proper construction of the review question, as it follows: (P) adults; (I) use of tDCS and/or rTMS; (C) simulated or false treatment; (O) reduction of altered neuropsychological symptoms; (S) controlled and/or randomized clinical trial to answer the following question: What is the influence of noninvasive neuromodulation on the performance of executive functions in overweight or obese individuals?
Inclusion criteria were: original articles from controlled and/or randomized clinical trials whose main subject matter was related to ETCC and/or rTMS in the treatment and/or rehabilitation of executive functions in adult humans, considering the context of variations in nutritional status. Incomplete studies, systematic reviews, articles outside the time frame of the research and studies without relevant results on diagnosis and rehabilitation of executive functions in obese individuals using ETCC and/or transcranial magnetic stimulation were excluded.
The selection was initially made by reading the titles and abstracts of the articles and applying the inclusion and exclusion criteria. A review was conducted by a pair of researchers (MMSLB and MRC) independently to ensure greater accuracy and credibility in selecting articles. In cases of disagreement, there was communication between the evaluators for a decision, by consensus, about the inclusion or not of the article. The study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42021261770.
The bias risk assessment tool proposed in the Cochrane Handbook for Systematic Reviews of interventions evaluated the included studies based on the criteria. These were used to minimize the risks of selection, performance, detection, wear, and reporting risks. Each item was scored as having low risk, uncertain risk, or high risk of bias. After analyzing each domain in the Cochrane Risk of Bias tool, each study was classified as having a general risk of bias, and the score was classified (22).
RESULTS
Using the descriptors above, 763 articles were identified in the databases defined for the search. After the selection and analysis of the studies found, 08 articles were selected for systematic review (Fig. 1).

Figure 1. Schematic representation of the study selection steps according to the methodological recommendations of the “Preferred Reporting Items for Systematic Reviews” (PRISMA). Flow diagram specific for systematic reviews.
Table I summarizes the studies selected to make up this systematic review. The main items were listed: author(s) and year of publication; study design, target population, sample size, type, and qualifiers of the proposed intervention (both the neuromodulation protocol and the tests for evaluating the executive function, comparator group, and primary outcomes).
Table I (cont.). Summary of the main findings in the selected articles.
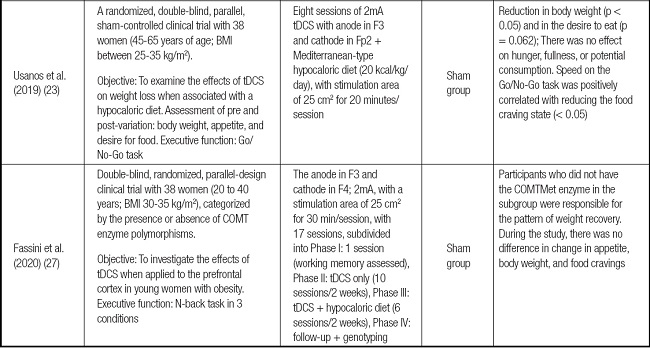
COMT: catechol-O-methyl transferase; Ctbs: TMS in excitatory theta-burst continuous mode; FP: flanker paradigm; RCT: randomized clinical trial; tDCS: transcranial direct current stimulation.
The results are organized according to the presentation of the sample variables (sample size, characteristics of the participants regarding age, gender, classification of global nutritional status), research design, type of intervention (with the description of procedures related to noninvasive neuromodulation and executive functions), type of control for the treatment group and characterization of primary outcomes (changes in overall nutritional status and functioning of the process evaluated in the participants, before and after the intervention).
The number of people included in the eight selected studies was 301 participants. The majority were female (235 participants), and 44 were left with an indeterminate gender definition. A survey sample size obtained an average of 30.1 participants per study (minimum of 12 and 76), as seen in table I.
There was variability in the age group of the participants in the different studies, as described below: 1) sample was composed of 38 women between 45 and 65 years (23); and 2) another sample included a smaller and younger selection (28 women between 19 and 22 years) (24), and we believe the difference between ages may have influenced the results.
The first demonstrated a favorable result of neuromodulation in reducing binge eating and increasing the agility of executive functions. At the same time, the second observed an increase in the number of calories ingested by participants and worse performance in inhibition control. The overall nutritional status varied between low weight and obesity grade 3, and the overall body mass index (BMI) average of the participants recruited by the selected studies was 28.1 kg/m2 (minimum of 19 and maximum of 40).
Regarding the type of stimulation modality, the proportion between noninvasive neuromodulation strategies as a form of treatment was 75 % (n = 6) with the use of tDCS (electric current) (23,25-29) and 25 % (n = 2) rTMS (magnetic pulses) (24,30).
Considering only the results of the articles that evaluated the effects of noninvasive neuromodulation in the modality of electric current (n = 6), there was the evaluation of the result after the application of anodic currents, with the most recurrent stimulation site being the left dorsolateral prefrontal cortex (F3) (83.3 %; n = 5) (23,25,28,29).
As for the studies that evaluated the effects of non-invasive neuromodulation using the magnetic pulse modality, in both studies the area of excitatory stimulation “theta-burst” was the left dorsolateral prefrontal cortex (F3) (24,30).
It is noteworthy that the study (25) sought to evaluate possible different results for the same result after the application of stimuli in other areas in the same group of participants, with the following assemblies: in F3 (anodic) with the cap on Fp2; in F4 (anodic) with the cathode in Fp2; and in F4 (cathodic) with the anode in the deltoid muscle. In the comparator group there was simulated stimulation (with the same settings mentioned above; however, there were only 30 seconds of active stimulation); in this group, only favorable results were obtained with anodic currents aiming at inhibition (interference control and selective attention).
The sites chosen for anodic direct current stimulation were: 1) F3 - 60 % (n = 3), using the Fp2 point as the cathode; 2) F4 and cerebellum - 20 % of studies (n = 1; each). In the TMS, the two studies used the same stimulation configuration: “theta-burst” mode in F3, with continuous trains of 600 pulses for 40 sec applied at 50 Hz.
Regarding the design of the study, half of the studies were of the clinical, randomized, parallel type (23,26,29), and the other half were of the cross-over type (24,25,28,30). Regarding blinding, 62.5 % (n = 5) were double-blind studies (23,25-27,29) and the rest (37.5 %) were single-blind studies (24,28,30).
Among all the studies selected, when considering the relationship between executive functions and binge eating and/or nutritional status, 62.5 % (n = 5) of the studies pointed to inhibition as the main way to influence the result of reduced binge eating and/or inhibitory control to resist impulsive behaviors/dietary temptations (23-26,30); the other studies (37.5 %) focused their interventions on observing changes in working memory before and after the intervention (27-29).
Regarding the instruments used to evaluate executive functions, in the included studies they were more frequent for the pre- and post-stimulation evaluation of inhibitory control (to measure interference control): flanker paradigm (PF) (24,30); binocular rivalry for continuous flash suppression (bCFS/NoCFS) (25); Stroop task (30), and Go/No-Go task (23,25).
To evaluate inhibition control (resistance to temptations and impulsive behaviors), only one study used visual tasks to elicit the ability to resist the foods shown, using questionnaires and visual analog scales to measure the variation in the intensity of inhibitory control (26).
Notably, only 37.5 % observed the influence of working memory response (27-29). However, considering that there are studies that have analyzed more than one aspect of executive function, including working memory and binge eating (food inhibition control) (25,27-29), the results exceed 100 % (n = 8). No article sought to establish the relationship between the influence of cognitive flexibility variability (via neuropsychological tests), before and after neurostimulation, in patients with binge eating, nor from the perspective of nutritional status.
After the interventions, the most commonly observed primary outcomes were described: reduction of food craving (23,26,29), improved performance in the active group from reduced response time, and increased accuracy in cognitive tasks (23,25,27,30).
On the other hand, the intervention group (participants who received real stimulation) showed increased hunger and food desire, as well as worsening working memory performance (28). In another study (30), the intervention group participants performed worse in the flanker paradigm (inhibiting and interference control). In addition, they had a higher calorie consumption when compared to shams.
The results of the methodological quality assessment for each of the 8 included studies are shown in table II. Five studies were considered to have a low risk of bias in all four domains: performance bias, detection bias, reporting bias, and trend data friction, as shown below (23,25,26,29,30). A high risk of bias was found in three studies. The risk was related to the researcher's allocation of participants and no random sequence generation or allocation concealment (selection bias) (27).
Table II. Summary of the analysis of the authors' judgments on each risk of bias domain for each included study.
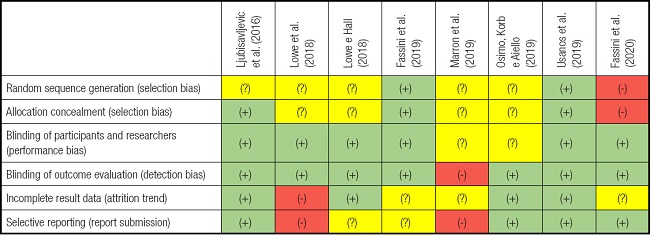
(+): low risk of bias; (-): high risk of bias; (?): uncertain risk of bias.
The tendency towards attrition and reporting bias was evidenced by the fact that one participant was allowed to have a longer interval between stimulations compared to that established and performed on the others (24). Furthermore, there was no blinding of results evaluation (detection bias) and selective reports were present (report bias) (28).
There was no specific mention in the above-mentioned studies on allocation concealment (selection bias), the method of blinding participants and researchers (performance bias), or the conditions of execution or values obtained after measurement. However, the authors mentioned that it was carried out.
DISCUSSION
Non-invasive neuromodulation influences the reduction of food craving and promotes improvement in the performance of executive functions, especially cognitive inhibition.
Regarding the relationship between age and performance of executive functions, a study with older participants (23) demonstrated the Go/No-Go task and a reduction of food desire. Differently, in a survey with young patients, an increase in the consumption of calories and worse performance in the flanker paradigm task were demonstrated. It should be noted that both used the same target (anodic at F3) but in different modalities: electrical and magnetic, respectively (24).
The use of non-invasive neuromodulation has consistently reduced food cravings, especially for sweet foods, fast food. However, a clear impact on changes in body weight has not been demonstrated. Furthermore, it improved performance of the participants' executive functions, especially in the groups of subjects with overweight or obesity (23,25-27,29).
The mechanism for weight loss is complex and involves other variables that are often not considered in studies, partly due to limitations in the methodological design itself or due to high costs that make genotyping unfeasible. Therefore, we recommend that during the clinical evaluation process of an obese person several factors can be considered as adjuvants in the weight loss process, from biological aspects (chewing, nutritional/dietary selection), to genetic study, to rehabilitation aiming at cortical biochemical adjustment (noninvasive neuromodulation).
The genotyping of the catechol-O-methyltransferase (COMT) enzyme showed that weight loss might be linked to specific enzyme polymorphisms, leaving the individual susceptible to easier weight recovery, resulting in higher energy expenditure (27).
COMT acts on the breakdown of catecholamines in the synaptic cleft, and is responsible for a large part of the metabolism of the neurotransmitter dopamine present in the prefrontal cortex. Therefore, this enzyme plays a vital role in the dopaminergic and noradrenergic balance. The COMT gene Val158Met polymorphism, caused by an amino acid exchange, promotes the phenotypic heterogeneity found in attention deficit hyperactivity disorder. There is evidence that it is involved in the emergence of disruptive energy behavior disorders, directly influencing food decisions. Higher appetite levels and weight loss were observed in participants with no polymorphism (29,31).
The food decision is a critical moment and precedes the oral processing of mastication or the process of bioavailability or storage of nutrients. It is conscious and dependent on brain inhibitory control, especially in the interference control modality (14,32).
Therefore, because executive functions are interdependent, from the change in self-regulation, memory inhibition can occur and vice versa, allowing individuals to suppress or ignore unwanted or outdated associations, thus helping them to use information relevant to dietary goals and disregard irrelevant information (33).
Judgment capacity and also executive functions are closely related to frontal lobe activity. This lobe is associated with the motor, perceptual and limbic regions, subdivided into four components: the premotor cortex, the primary motor cortex, the prefrontal cortex, and the supplementary motor area. Five major functional areas are recognized for the prefrontal cortex: dorsolateral prefrontal (CPFDL), ventrolateral (CPFVL), orbitofrontal (CPFOF), ventromedial (CPFVM), and anterior cingulate (ACC) cortex (10,34).
Highlighted cortical areas and correlated actions/functions, reasoning, social control of behavior, and action planning, are related to the CPFVL and CPFOF regions (10), while the modulation of emotions and control of attentional processes are related to the CCA. The CPFVL region is involved in working memory, but there is a input from the CPFDL region during manipulation of information.
The network functioning of cortical functions provides the substrate for choosing which test is the most appropriate, considering the process or subfunction to be evaluated. The N-back task, for example, aims to bombard working memory using increasing memory spans (items that must be retrieved shortly after successive updating of similar information). The letter ‘N' reveals the number of spans that will be used: 0-back, the stimulus presented in the current one; 1-back, the stimulus presented on the previous screen; 2-back, the stimulus given two screens back, and so on (35).
On the other hand, the Go/No-go tasks and the flanker paradigm (FP) require a response selection process with execution/inhibition of the motor response, triggered by a movement of pressing or not pressing a key. The task requires agility and a high cognitive level between response selection, decision making, or attention and response inhibition (36,37).
In the context of the influence of executive functions, there is a relationship between impulsivity derived from low performance of inhibitory control influencing the low oral processing of food. Cognitive functions are highly influenced by reversible biological processes such as nutritional intervention and traditional speech therapy, concomitant with non-invasive neuromodulation (38).
LIMITATIONS AND STRENGTHS
A limitation of this study was the impossibility of performing a meta-analysis due to lack of data in the articles. It was possible to apply specific statistical tests. The mastication efficiency metric was another point that deserved discussion and was not considered in any article. This is because overweight and individuals with obesity tend to have changes in masticatory behavior, which can contribute to more difficulties in performing masticatory functions when compared to eutrophic individuals.
Furthermore, individuals with obesity tend to have a chewing pattern with few chewing cycles, which in turn leaves the surface area for the action of digestive enzymes smaller, which can decrease the efficiency of the digestive and absorptive process, reducing satiety and increasing signaling for a pattern of hunger, since digestibility is involved in the control of the glycemic response. One hunger and satiety control mechanism involves glucostatic management (32,39,40).
CONCLUSION
This systematic review showed that neuromodulation generates changes in food desire (decrease or increase) and can influence executive functions in people with obesity. However, due to the heterogeneity found in the assembly of the stimulation protocols used, as well as the different executive functions evaluated in the studies included in this work, there is a need for additional research using masticatory efficiency measures concomitant with the food desire profile, as well as the evaluation of all executive functions to highlight which component prevails in food decisions.

















