INTRODUCTION
Vitamin D is currently attributed a pleiotropic profile (1-3). In point of fact, basically every human tissue and cell contains vitamin D receptors, and its biological effects are categorized as skeletal (bone metabolism and calcium homeostasis) and extra-skeletal (hypovitamosis D appears to be involved in autoimmune diseases, infections, neuropsychiatric disorders, cardiovascular risk, prostate and breast cancer, etc.), a circumstance that justifies the interest in monitoring its body content.
Furthermore, the prevalence of childhood obesity has gradually increased in the course of the last decades, establishing itself as the most relevant nutritional disorder in our environment (4,5). Even though obesity is considerate as a multifactorial disorder, the celerity of its increase in prevalence is related essentially to environmental factors: scarce healthy nutrition habits as well as a sedentary lifestyle conditioned, to some extent, by new technologies (screen time, including television viewing, use of computers and video games) (6).
Several studies have demonstrated that obesity childhood is related to vitamin D deficiency (7-10). The main source of vitamin D is the exposure to natural sunlight (cutaneous synthesis through ultraviolet B radiation) and, therefore, the higher prevalence of vitamin D deficiency in adolescents with obesity could be related to a more sedentary lifestyle (less mobility and participation in outdoor activities) and, consequently, a lack of adequate sun exposure. However, many explanations have been proposed for this association, but interestingly, they hardly introduce theoretical mechanisms that imply limited sun exposure: storage or sequestration in adipose tissue, volumetric dilution, impaired hepatic 25-hydroxylation, etc. (33,11 12 1311-1414).
The main causes of vitamin D deficiency are generally related either to some physical agent that obstructs solar radiation (clothing, sunscreen, etc.) or to geographical characteristics, such as latitude and season of the year, cloudy weather, altitude, etc. (2,15). In fact, recent studies using an objective and accurate method for ultraviolet radiation monitoring in adolescents have revealed that rural residents received higher levels of ultraviolet radiation exposure than urban residents did (16,17).
This study aims to compare vitamin D status between adolescents with obesity living in an urban area and in a rural area in Navarra, Spain (latitude between 43° 16' 42” and 41° 55' 22” North). We hypothesized that environmental factors (outdoor activities and sun exposure) would be decisive in reducing the body content of vitamin D in patients with obesity.
METHODS
PARTICIPANTS
We conducted a cross-sectional study (convenience sample) in a group of 259 patients, aged 9.6-15.7 years, previously diagnosed with obesity (obesity group, BMI-DS > 2.0, 97th percentile) and 249 patients, aged 9.8-15.3 years, diagnosed with severe obesity (severe obesity group, BMI-DS > 3.0, 99th percentile), who underwent clinical examination (sex, age, season of study visit, place of residence and BMI) and blood testing (calcium, phosphorus, calcidiol and PTH) in the Pediatric Endocrinology Unit during the period January 2014 to December 2021. The place of residence was categorized as urban or rural subgroups by means of population (more or less than 10,000 inhabitants, respectively).
In addition to that, these parameters (clinical examination and blood testing) were determined in a control group that consisted of 251 healthy adolescents, aged 9.9-15.4 years, with BMI-DS ranging from -1.0 (15th percentile) to +1.0 (85th percentile).
All participants included in the study showed any sign of pubertal development (Tanner stages II-V), and were additionally Caucasian individuals living in Navarra, Spain. Participants had neither any condition affecting bone health or chronic pathologies that might alter growth, body composition, food ingestion, or physical activity, nor had they received any medication (antiepileptic drugs or glucocorticoids), vitamin D, or calcium supplements.
Parents and/or legal guardians were informed and provided written consent for the participation in this study in all cases. This study was approved by the Ethics Committee for Human Investigation of Navarra Hospital (in accordance with the ethical standards laid down in the 1964 Declaration of Hensinki and later amendments).
CLINICAL EXAMINATION
The standardized protocol that was used for the anthropometric measurements had been previously published (18). Weight and height measurements were taken with subjects in underclothing and barefoot. Weight was measured using an Año-Sayol scale (reading interval 0-120 kg and a precision of 100 g), and height was measured using a Holtain wall stadiometer (reading interval 60-210 cm, precision 0.1 cm).
The standard deviation (DS) values for the BMI were calculated applying the program Aplicación Nutricional, from the Spanish Society of pediatric gastroenterology, hepatology and nutrition (Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica, available at http://www.gastroinf.es/nutritional/). The graphics by Ferrández et al. (Centro Andrea Prader, Zaragoza 2002) were used as reference charts (19).
BLOOD TESTING
Blood testing for biochemical determinations (calcium, phosphorus, calcidiol and PTH) was collected under basal fasting conditions (between 8:00 h and 9:00 h after an overnight fast).
Calcium and phosphorous plasma levels were determined in a fasting sample of blood using colorimetric methods in a COBAS 8000 analyzer (Roche Diagnostic, Mannheim, Germany). The determination of calcidiol levels required a high-specific chemiluminiscence-immunassay (LIAISON Assay, Diasorin, Dietzenbach, Germany), and the determination of PTH levels a highly specific solid-phase, two-site chemiluminescent enzyme-labeled immunometric assay in an Immulite analyzer (DPC Biermann, Bad Nauheim, Germany).
The criteria of the United States Endocrine Society (20) were followed to distribute individuals according to vitamin D plasma levels. In this way, calcidiol plasma levels lower than 20 ng/ml (< 50 nmol/L) corresponded to vitamin D deficiency, calcidiol levels between 20 and 29 ng/ml (50-75 nmol/L) to vitamin D insufficiency, and concentrations equal to or higher than 30 ng/ml (> 75 nmol/L) to vitamin D sufficiency.
STATISTICAL ANALYSIS
Results are displayed as percentages (%) and means (M) with corresponding standard deviations (SD). The statistical analysis (descriptive statistics, Student's t-test, analysis of variance, χ2 test, and Pearson's correlation) was performed using the program Statistical Packages for the Social Sciences version 20.0 (Chicago, IL, USA). Statistical significance was assumed when the p-value was < 0.05.
RESULTS
Table I shows and compares the distribution of demographic features in the control, obesity and severe obesity groups. No significant differences were found in the distribution in relation to sex, season of blood sample collection and place of residence.
Table I. Distribution of geographic/demographic features in severe obesity, obesity, and control groups.
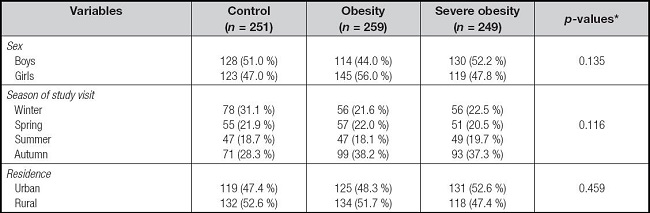
*Chi2.
The mean values for calcidiol were significantly lower (p < 0.001) in the obesity (22.1 ± 8.3 mg/mL) and severe obesity (19.3 ± 7.9 mg/mL) groups than in the control group (27.4 ± 7.5 mg/mL), whereas the mean values for PTH levels were significantly higher (p < 0.001) in the obesity (49.6 ± 17.3 ng/mL) and severe obesity (51.2 ± 16.8 ng/mL) groups than in the control group (30.1 ± 13.8 ng/mL). There were no significant differences in the variables of age (control group: 12.8 ± 1.6, obesity group: 13.1 ± 1.7, and severe obesity group: 13.2 ± 1.7 years; p = 0.512) and calcium (control group: 9.9 ± 0.3, obesity group: 9.8 ± 0.3, and severe obesity group: 9.7 ± 0.3 mg/dL; p = 0.441) and phosphorus levels (control group: 4.4 ± 0.5, obesity group: 4.4 ± 0.6, and severe obesity group: 4.4 ± 0.5 mg/dL; p = 0.740) between the different groups. Obviously, the mean values for BMI-SD were significantly higher (p < 0.001) in the severe obesity (4.17 ± 1.11) and obesity groups (2.49 ± 0.27) with respect to the control group (0.14 ± 0.09).
The prevalence of vitamin D deficiency was significantly higher (p < 0.001) in the severe obesity (55.0 %) and obesity (37.1 %) groups than in the control group (14.0 %). Furthermore, only 9.2 % and 15.1 % of patients of the severe obesity and obesity groups showed levels of calcidiol higher than 30 ng/mL, respectively, in contrast to 40.4 % of the participants in the control group.
In each group, the highest prevalence of vitamin D deficiency (p < 0.001) corresponded to winter (severe obesity group: 71.4 %, obesity group: 41.1 %, and control group: 25.6 %), and they reached a minimum in the summer (severe obesity group: 32.7 %, obesity group: 25.5 %, and control group: 2.2 %). The prevalence of vitamin D deficiency in the different seasons of the year was significantly higher (p < 0.001) in the severe obesity and obesity groups with respect to the control group.
Table II shows and compares the mean values for biochemical determinations on the basis of vitamin D status between the different groups. No significant differences were detected in calcium and phosphorus levels between the different vitamin D status, and obviously calcidiol levels were significantly lower (p < 0.001) in vitamin D insufficiency and deficiency participants than sufficiency vitamin D in each groups. PTH levels were significantly higher (p < 0.001) in vitamin D insufficiency and deficiency participants than in vitamin D sufficiency within each group. In addition, there were no significant differences in calcium, phosphorus, and calcidiol levels in each vitamin D status between the different groups. However, PTH levels were significantly higher (p < 0.001) for each vitamin D status in the severe obesity and obesity groups with respect to the control group.
Table II. Biochemical determinations according to vitamin D status in the control, obesity, and severe obesity groups (M ± SD).

*ANOVA;
†ANOVA between groups (p < 0.001).
Figure 1 exposes and compares the prevalence of vitamin D deficiency in relation to the place of residence between control, obesity and severe obesity groups. In the control group, there were significant differences (p < 0.01) in vitamin D deficiency between urban (21.2 %) and rural (7.6 %) subgroups. In the obesity group, vitamin D deficiency was significantly more frequent (p < 0.01) in the urban (51.2 %) than in the rural subgroup (23.9 %); additionally, in the severe obesity group, also vitamin D deficiency was significantly more frequent (p < 0.01) in the urban (67.2 %) than in the rural subgroup (41.5 %).
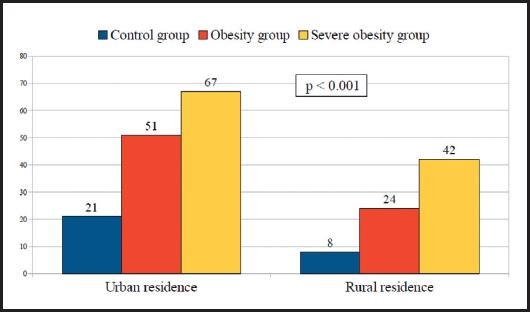
Figure 1. Prevalence (%) of vitamin D deficiency in relation to the place of residence in the control, obesity, and severe obesity groups.
Figure 2 displays and compares the prevalence of vitamin D deficiency according to the seasons of the year between the participants in the control, obesity and severe obesity groups that lived in urban residence. In the control group, there were significant seasonal variations (p < 0.01) in vitamin D deficiency which showed the lowest prevalence of vitamin D deficiency during the summer (6.3 %) and the highest during the winter (40 %). In contrast, there were no significant seasonal variations in the prevalence of vitamin D deficiency throughout the year in both the severe obesity and obesity groups. In fact, in severe obesity group the prevalence of vitamin D deficiency during the summer was 66.7 % and during the winter 71.8 % (p = 0.825); within the obesity group, vitamin D deficiency was 52.9 % in the summer and 53.6 % in the winter (p = 0.633). The prevalence of vitamin D deficiency in the different seasons of the year was significantly higher (p < 0.01) in the severe obesity and obesity groups with respect to the control group.
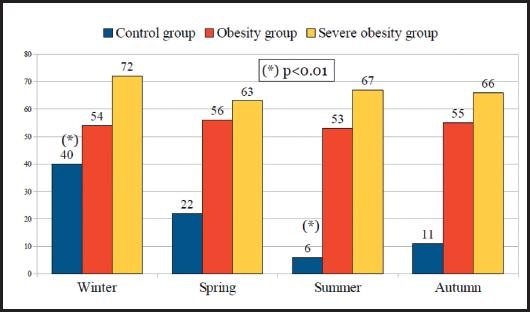
Figure 2. Prevalence (%) of vitamin D deficiency according to the seasons of the year among the participants in the control, obesity, and severe obesity groups who lived in an urban area.
Figure 3 show and compares the prevalence of vitamin D deficiency according to the seasons of the year between the participants in the control, obesity and severe obesity groups that lived in rural residence. All groups presented significant seasonal variations in vitamin D deficiency throughout the year. In each group, the lowest prevalence of vitamin D deficiency corresponded to summer, and they reached a maximum in the winter. In severe obesity group, the prevalence of vitamin D deficiency during the summer was 7.1 % and during the winter 70.6 % (p < 0.01). In obesity group, vitamin D deficiency was 10 % in the summer and 35.3 % in the winter (p < 0.01). And, finally, in the control group, vitamin D deficiency was 0.0 % during the summer and 16.7 % during the winter (p < 0.01). The prevalence of vitamin D deficiency in the different seasons of the year was significantly higher (p < 0.01) in the severe obesity and obesity groups with respect to the control group.
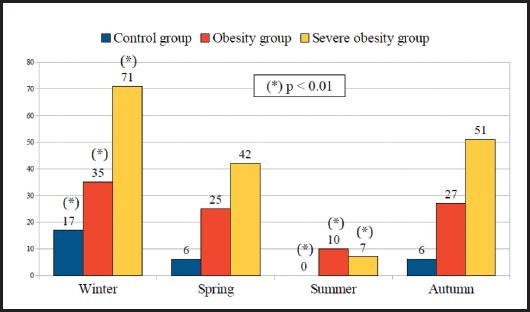
Figure 3. Prevalence of vitamin D deficiency according to the seasons of the year among the participants in the control, obesity, and severe obesity groups who lived in a rural area.
There was a negative correlation (p < 0.01) between calcidiol and PTH levels (r = -0.383). In addition, there was also a positive correlation (p < 0.01) between PTH and BMI-SD (r = 0.377) and a negative correlation (p < 0.01) between calcidiol and BMI-SD (r = -0.388).
DISCUSSION
This study verifies that vitamin D deficiency is a common in adolescents with obesity. Furthermore, our data suggest that this higher prevalence of vitamin D deficiency in these patients could be ascribed to inadequate sunlight exposure, since there was a weaker trend to vitamin D deficiency in those patients living in rural areas than in those living in urban areas.
The higher prevalence of vitamin D deficiency in patients with obesity has been sustained by several studies (7-10), even though the potential mechanisms for this association still remain questionable. Nevertheless, at present, the most qualified hypotheses about the inverse relationship between vitamin D deficiency and obesity refer either to storage or sequestration of vitamin D in adipose tissue or volumetric dilution of vitamin D. Clinical studies have shown that obesity does not affect the cutaneous synthesis of vitamin D, but as it is a fat-soluble vitamin, it is accumulated and retained in adipose tissue (storage site or sequestration hypothesis). And, therefore, the greater the storage capacity of this vitamin in adipose tissue (severe obesity and obesity groups), the lower the serum levels of calcidiol (21,22). In fact, we found that calcidiol levels in the participants included in this study (severe obesity, obesity and control groups) were inversely correlated with body mass index; this is an anthropometric measurement that has been frequently used in the diagnosis and follow-up of adolescents with obesity since it shows a good correlation with body fat content (23). A second probable mechanism of the inverse relationship between vitamin D deficiency and obesity could be a volumetric dilution; that is, vitamin D would be distributed in body compartments that increase with obesity (serum, fatty tissue, liver, etc.), thereby making serum levels lower (11,13). It has also been suggested that lower levels of calcidiol in patients with obesity could be due to impaired hepatic 25-hydroxylation related to non-alcoholic fatty liver disease, a condition that is common in adults with obesity but less frequent in childhood obesity (24). However, none of the previously mentioned hypotheses would explain by itself, for example, the stronger trend to vitamin D deficiency in patients with obesity (severe obesity and obesity groups) living in urban areas than in those living in rural areas, as we identified in this study.
Vitamin D receptors are present in a large variety of tissues and cells in the body (muscle, heart, blood vessels, neurons, immune cells, breast, colon, prostate, etc.), and this fact supports the biological importance of sufficient calcidiol serum levels (1,20). Moreover, adipose tissue also expresses vitamin D receptors, and 1α-hydroxylase enzyme locally converts calcidiol to calcitriol (biological active form of vitamin D), and that process is not regulated by parathyroid hormone, in contrast with renal 1α-hydroxylase (25). Additionally, some experimental data support that vitamin D could have an anti-obesity effect by inhibiting adipogenesis during early adipocyte differentiation and independently of PTH. That is, vitamin D might be implicated in the pathogenesis of obesity, rather than being a consequence (3,14). These findings suggest, on one side, that adipose tissue could play a role in vitamin D metabolism rather than being a passive store of fat soluble nutrients, and, on the other side, that a bidirectional causal relationship between vitamin D deficiency and obesity cannot be excluded. However, several studies have shown no effect of vitamin D treatment on reducing body weight and/or body composition, suggesting that although vitamin D deficiency is associated with obesity, it is not bidirectional (26,27).
In accordance with most authors (7,8,10), we found a negative correlation between PTH and calcidiol levels, and this would be consistent with the physiological feedback mechanism of vitamin D on PTH secretion. But, interestingly, it is worth noting our finding that PTH levels were also significantly higher—independent of vitamin D status—in the patients with obesity (severe obesity and obesity groups) with respect to the control group. Many researchers have postulated that this elevation of PTH might increase calcium influx into adipocytes, which then leads to increased lipogenesis and potentially reduces catecholamine-induced lipolysis and, consequently, fosters fat storage (28). Additionally, several observational studies have shown that PTH levels in obesity are independent of vitamin D status and it does not represent, as is commonly assumed, secondary hyperparathyroidism from hypovitaminosis D (29). However, despite the above biological assumptions that obesity is related with vitamin D deficiency and elevated PTH levels, the reason given for this association remains unexplained. In fact, some authors are currently questioning whether vitamin D deficiency and elevation of PTH is a consequence or cause of obesity (14), or whether this association is causality or casualty (3).
Obviously, unhealthy eating habits are related to childhood obesity, and this entails a lower intake of vitamin D. However, the main source of vitamin D is exposure to natural sunlight, while approximately 10 % comes from natural dietary sources (1,2). Few foods naturally contain vitamin D (oily fish such as salmon, sardines, mackerel, and tuna, as well as shiitake mushrooms and eggs yolk) and, depending on the country, additional sources include fortified foods such as dairy products, orange juice, breakfast cereals, cookies and butter or margarine (2). Therefore, even though diet seems to be probably an irrelevant factor in the acquisition of optimal levels of vitamin D, it could not be completely excluded.
Because geographical conditions affect body vitamin D content, we cannot refer to a vitamin D status in a determined population without mentioning them. In our case, it should be noted that Navarre is a Spanish region located in the north of the Iberian peninsula with a population of 661,537 inhabitants (2021 census, National Institute of Statistics), 58.1 % of whom live in urban areas and 41.9 % in rural areas. Besides, it is characterized by a high frequency of precipitations and/or cloudiness and, especially, a high latitude (between 41° 55' 22” and 43° 16' 42” North). When the zenith angle of the sun is oblique, as occurs in the winter months in both hemispheres, type B ultraviolet radiation barely reaches the earth's surface above and below 40° N and 40° S latitude, causing a very low or absence of cutaneous synthesis of vitamin D, even with prolonged sun exposure (15,20). In compliance with several studies (7,30), this is a potential explanation for the seasonal variations in the prevalence of vitamin D deficiency (maximum prevalence in the winter months and minimum in the summer months) that we found in the control group.
Recent studies using personal electronic ultraviolet radiation dosimeters have displayed higher ultraviolet radiation exposure in children and adolescents living in rural areas compared to those living in urban areas due to differences in types of activity. Children and adolescents living in rural areas spend more time after school and during weekdays practicing outdoors chores during peak ultraviolet radiation hours (10 am-4 pm), compared to those living in urban areas who spend more time participating in indoor sports and/or leisure activities and, therefore, reducing exposure to sunlight (16,17). These data allowed us to hypothesize a much simpler explanation for the relationship between obesity and vitamin D deficiency: environmental factors (outdoor activities and sun exposure) would be determining in reduced body content of vitamin D in patients with obesity.
Indeed, we also found seasonal variations in the prevalence of vitamin D deficiency (maximum prevalence in the winter months and minimum in the summer months) in patients with obesity (obesity and severe obesity groups), although showing significantly lower values with respect to the control group. That is, on one hand, this would confirm that sunlight exposure has a large impact on vitamin D status also in patients with obesity and, on the other hand, we found a stronger trend to vitamin D deficiency in patients with obesity (obesity and severe obesity groups) living in urban areas than in those living in rural areas. Nevertheless, the most remarkable finding of this study was that patients with obesity (obesity and severe obesity groups) living in urban residence did not present significant seasonal variations in vitamin D deficiency throughout the year in contrast to those patients with obesity (obesity and severe obesity) living in rural areas of residence, who presented a maximum prevalence of vitamin D deficiency in the winter months and a minimum in the summer months. Therefore, these findings would support the hypothesis that the greater tendency to present vitamin D deficiency in adolescents with obesity would be related to a sedentary lifestyle and, consequently, to lack of adequate sun exposure.
At present, and despite the hypotheses recounted above, vitamin D photobiology suggest that the most probable mechanism for vitamin D deficiency in adolescents with obesity are environmental factors (reduced sunlight exposure) rather than metabolic alterations (sequestration in adipose tissue, volumetric dilution, impaired hepatic 25-hydroxylation, etc.), as our findings outline. However, other mechanisms cannot be completely excluded, as they may contribute concurrently.














