INTRODUCTION
Restrictions resulting from the COVID-19 pandemic have challenged the world to live in a new reality. In contrast to other European countries where the main concern was to take care of the elderly population, in America, the main concern was people with comorbidities such as obesity, diabetes, and hypertension. Especially early-onset diabetes and obesity increase the risk of hospitalization, Intensive Care Unit (ICU) admission, and intubation (1). The interdisciplinary approach to treating these patients has changed due to the high risk of infection. In the case of nutritional therapy, the guidelines recommend the minimum possible contact, and all the nutritional information was obtained from the patient’s family, nurses and physicians (2,3).
As in other parts of the world, the first months were uncertain in terms of medical and nutritional treatment. However, thanks to the publications of other researchers and the experiences of medical teams, the first international guidelines were released to improve clinical practice. Most patients with severe COVID-19 required intubation and therefore, enteral nutrition (EN) was provided. It is essential to highlight that all guidelines established that although nutritional requirements should not be entirely fulfilled at the beginning, they should be met after the first week of nutritional intervention.
In the acute phase, the recommendation is to start with a prescription of 15-20 kcal/kg actual body weight (ABW), which may represent around 70 % of the energy requirements, and after the fourth day in the ICU, it should be increased to 25-30 kcal/kg ABW in patients who have normal body mass index (BMI) and 11-14 kcal/kg ABW in patients who live with obesity; if BMI is greater than 50 kg/m2, 22-25 kcal/kg of ideal body weight (IBW) can be used. Protein intake must be 1.2-2.0 g/kg ABW unless BMI is greater than 50 kg/m2, which is when 2.0-2.5 of IBW must be used (2,4,5). Furthermore, the importance of having an adequate infusion of calories to improve the outcomes of hospitalized patients is highly recognized (6). The specific macro and micronutrients infused through the nutritional support or drugs (propofol or intravenous fluids) can impact the patient outcome.
Many of these recommendations were released during data collection. Thus, it was of interest to investigate if the prescriptions of nutritional support given by EN were adequate. The optimal amount was defined as 80 % of the 24-hour calorie target throughout a patient’s hospital stay (7,8), following the recommendations of the international guidelines for treating a critically ill patient. Our hypothesis proposes that even if energy prescription could be optimum according to international guidelines, the infusion of it could be deficient due to the lack of follow-up by the nutritional team and that better adequation could lead to lower mortality risk. Therefore, this study aimed to provide an overview of the nutritional support given by EN and identify if it reaches the dietary recommendations provided by the international societies. Additionally, a secondary aim was to determine if any specific nutrients may impact the mortality in hospitalized patients with COVID-19 at a third referral hospital.
MATERIAL AND METHODS
We performed a cohort study involving adult patients with COVID-19 who were admitted to a third referral hospital (April-June 2020). This period was chosen for two reasons. First, it was just at this time that the first recommendations for the nutritional management of patients with COVID-19 came out, and even with the personnel restrictions, we attempted to evaluate the effectiveness of the response to the health emergency in this area. Second, it was the first trimester after the hospital was converted into an exclusive COVID-19 treatment centre. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving humans were approved by the Institutional Review Board.
The nutritional risk was assessed using the Nutrition Risk Screening (NRS) 2002 (11,12) score during the first 24 hours of hospital admission. The NUTRIC score was recorded only for those who entered the critical areas (13). Regarding anthropometric variables, during the admission triage, the height and weight of all patients were measured by a medical doctor if their condition allowed it; otherwise, the weight referred by the patient or family member was recorded. In consequence, the calculation of nutritional requirements and adequacy were set with ideal weight (2,4,5,14).
The biochemical parameters and the clinical data recorded were comorbidities, gastrointestinal symptoms related to COVID-19 (dysgeusia, diarrhea, nausea, and vomiting), the severity of disease (from computerized tomography), the National Early Warning Score (NEWS) (which is a screening tool) (15,16), the length of stay (LOS) in hospital, invasive mechanical ventilation (MV), and mortality. Additionally, for those in the critical areas, the Sequential Organ Failure Assessment Score (17) and Acute Physiology and Chronic Health Evaluation (18) score were assessed. For those who had MV, the Yale Swallow Protocol (19) and a volume-viscosity swallow test (V-VST) (20) were performed after extubation.
The dietetic variables included feeding at admission. For those who required EN, the prescription and infusion of EN (including total and by kg of IBW) were recorded as follows: days 0 (day of colocation of enteral access using nasogastric tube feeding [NGT] or gastrostomy), 1, 2, 3, 7, 12, and 14 and when post-extubated (P-E) (only those who required EN due to failing all consistencies in the V-VST). The registration of prescription and infusion was performed during the first 24 hours. The type of formula (polymeric, specialized [different formulas modified in their composition to have specific nutrients that include specific nutrients as n-3 poly unsaturated fatty acids or PUFA, arginine, fiber, low in specific electrolytes, or semi-elemental formula] or protein module) and specific nutrients derived from them (such as n-3 PUFA, arginine, fiber, vitamins, and minerals) were noted. We also accounted for the infusion of intravenous solutions that provide energy (such as propofol and glucose solutions of 5 % and 50 %) while considering them as non-nutritional calories (NNC) to determine the cumulative caloric and protein balance and nutritional adequacy according to the international guidelines (2,21).
STATISTICAL ANALYSIS
The descriptive analysis was performed using central tendency measures and the type of distribution of each variable with the help of the Kolmogorov-Smirnov normality test. To identify group differences according to their type of feeding, for the quantitative variables Mann-Whitney’s or t-tests for independent samples were performed, while for qualitative variables, the Chi-squared test was used with a significance value set as ≤ 0.05. Subsequently, regressions were performed for longitudinal data for random effects of the protein and energy intake administered to patients. This was done to identify significant differences between the participants who died during their hospital stay and those who left home while considering the time from the day of their admission to the hospital until their discharge. Likewise, the biochemical variables were related to caloric consumption, particularly those calories that did not come from food (NNC), to identify those that could modify the outcomes of hospital stay time, MV time, and death of the participants using a significance value ≤ 0.05. Finally, based on the previous results, a random-effects analysis of the parametric survival-time model was used to identify and quantify the risk of each variable for dying, and the Hausman test was used to confirm the model.
RESULTS
A total of 835 patients were screened. At hospital admission, the oral route was the most recurrent prescription (71 %); however, the most severe patients stayed fasting (29 %) to be stabilized or intubated as soon as possible. Out of the admitted patients, only 229 were followed, as they required nutritional support with EN (27.6 %). They had an average MV duration of 13.1 ± 9.8 days, 57.6 % died, and 106 were discharged from the hospital (Fig. 1). Table I shows the demographic characteristics of these patients compared to those who maintained oral food intake.
Table I. Baseline characteristics of patients.

Patients are divided into sthose who feed orally and those who require enteral nutrition.
t-tests for independent samples were used for quantitative variables, while Chi-squared was used for qualitative variables.
The significant p-value was set at ≤ 0.05
A total of 75.7 % of patients were admitted with nutritional risk, with a reduction in food intake in the week before hospitalization of 66.8 ± 25.5 % of the usual intake. When comparing the NRS-2002 score obtained at admission (3 [IQR 2-4]) and when entering critical areas due to the need for MV (3 [IQR 4-6]), a significant difference was found (p < 0.001). Regarding gastrointestinal symptoms related to COVID-19, 10 % had vomiting, 20 % had diarrhea, 10 % had nausea, and 10 % had dysgeusia. Only vomiting was associated with a decreased food intake (R -0.144; p < 0.001).
Patients had the following anthropometric measurements: the height was 1.65 ± 0.138 m, current weight was 81.5 ± 16.9 kg, and BMI was 29.4 (26.6-33.2) kg/m2. The most relevant biochemical indicators were the following: 3.52 ± 0.46 mg/dl albumin; 174.5 ± 93.2 mg/dl glucose; 1.12 ± 0.51 mg/dl creatinine; 167.4 ± 81.1 mg/dl triglycerides; 20.2 ± 9.6 mg/dl PCR; 969.2 ± 869.5 ng/ml ferritin; 21.7 ± 8.27 ng/ml vitamin D; and 2.22 ± 2.04 mmol/l lactate.
The access route for the infusion of EN was the NGT or gastrostomy even in those with MV, with continuous infusion for 24 hours. The most commonly prescribed type of formula was a polymeric formula (56.0 %), followed by a specialized formula (41.1 %). Finally, only a few were prescribed an exclusive protein module (2.9 %). Of the patients who required a P-E swallowing test, 20 % continued with EN, 67 % were prescribed an oral diet, and 13 % fasted with only an IV glucose solution.
On the first two days, energy infusion achieved more than 80 % adequacy (11.7 ± 4.9 kcal/kg); however, on day 14, the adequacy rate was less than 60 % (25.4 ± 7.4 kcal/kg and when P-E 26.8 ± 6.1 kcal/kg). As for protein, it was possible to have an infusion greater than 75 % on the first days of enteral feeding infusion (1.3 ± 0.3 g/kg); however, after being P-E, the infusion did not reach more than 50 % (1.5 ± 0.4 g/kg).
The energy intake of NNC oscillated around 400 kcal per day, which corresponded to 89 % on the first day and decreased in percentage by increasing the contribution through EN to 40 % of the total energy on average in the following days (Fig. 2A). This is also reflected in the macronutrient proportions since, on the first days, the infusion of lipids represented about 70% of energy consumption; however, on day 14, the proportion of macronutrients changed to 43.7 ± 30.9 % for carbohydrates, 17.6 ± 11.3 % for protein, and 32.2 ± 23 % for lipids (Fig. 2B). Only patients who received specialized formulas received n-3 PUFA and arginine within their nutritional content. The average infused n-3 PUFA and arginine during the first 15 days was 1.72 g (IQR: 0.8-4.0) and 6.77 g (IQR: 2.75-11.9), respectively. Additionally, the contribution of other micronutrients was recorded, which confirmed that the recommended daily doses were reached. Table II shows these nutrients along the days and the biochemical parameters showing differences in all time groups.
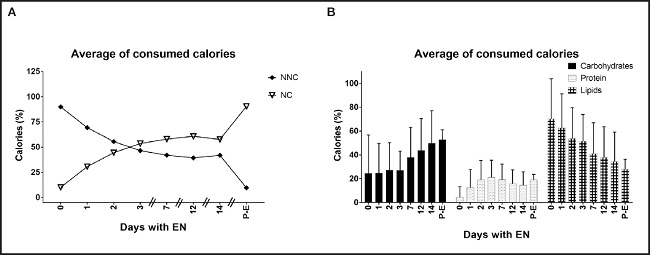
Figure 2. Average of consumed total calories. Post-E: post-extubated; NNC: non nutritional calories; NC: nutritional calories. A. Average of consumed calories from different sources. Represents the amount of calories subjects consumed from nutritional support (NC) and other sources such as propofol and glucose (NNC). Random-effects multi-way ANOVA. B. Macronutrient proportion of total energy infused. Represents the percentage of carbohydrates, protein and lipids from the total energy intake. Random-effects multi-way ANOVA.
Table II. Changes throughout time in nutrient consumption and biochemical parameters.

n-3 PUFA: n-3 poly unsaturated fatty acids; BUN: blood urine nitrogen; TG: triglycerides; CRP: C reactive protein; P-E: post-extubation; SD: standard deviation.
ANOVA analysis was performed. *The significant p-value was set at ≤ 0.05
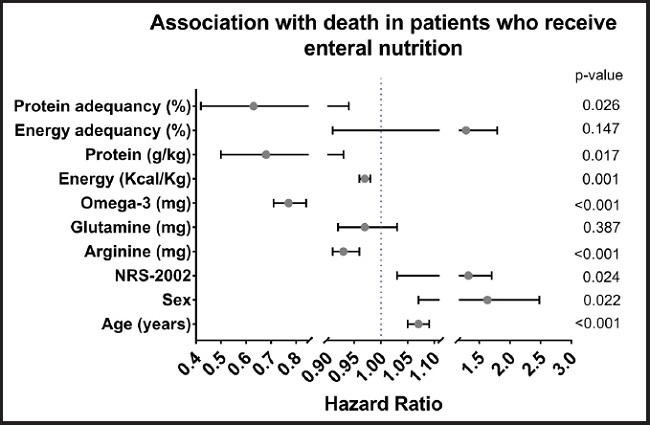
Linear regression models by random effects were used to find associations of specific variables, obtaining the following results: the days of LOS in the hospital and days of MV increased with each calorie infused (LOS: coefficient 0.002, 95 % CI: 0.001 to 0.004, and p < 0.001; MV: coefficient 0.001, 95 % CI: 0001 to 0.002, and p < 0.001) and with the administration of propofol (ml) (LOS: coefficient 0.04, 95 % CI: 0.01 to 0.072; MV: coefficient 0.03, 95 % CI: 0.01 to 0.05, and p = 0.003). An association was found between increased triglycerides and calories infused (coefficient 0.07, 95 % CI: 0.04 to 0.10, and p < 0.001), between CRP reduction and calories infused (coefficient -0.005, 95 % CI: -0.007 to -0.003, and p < 0.001), and between increasing CRP and propofol infusion (coefficient 0.13, 95 % CI: 0.06 to 0.20, and p < 0.001). The variables studied in patients with EN that were related to higher mortality were found to be age (HR: 1.07; 95 % CI: 1.05 to 1.09), sex (HR: 1.63; 95 % CI: 1.07 to 2.48), and nutritional risk (HR: 1.32; 95 % CI: 1.03 to 1.70). On the other hand, those associated with lower mortality were infused energy (kcal/kg) (HR: 0.97; 95 % CI: 0.96 to 0.98), infused protein (g/kg) (HR: 0.68; 95 % CI: 0.50 to 0.93), the percentage of protein adequacy (HR: 0.63; 95 % CI: 0.42 to 0.94), and the contribution of specific nutrients like arginine (HR 0.93; 95 % CI: 0.91 to 0.96) and n-3 PUFA (HR 0.77; 95 % CI: 0.71 to 0.84) (Fig. 3).
DISCUSSION
Healthcare providers had to create new strategies to give adequate attention to COVID-19 patients. In the field of nutrition, one of the biggest challenges was the incapacity of weight measurement due to the gravity of their condition or the access restriction to the areas. Demographic and clinical characteristics were similar to those previously reported in the literature, showing that age, being male, comorbidities, and nutritional status are important risk factors of mortality (9,22-24).
Other studies have demonstrated the great utility of the NRS-2002 score identifying the nutritional risk in patients with COVID-19 (25,26). For the majority of our patients, the nutritional risk relied on the reduction of food intake; as we showed, due to the disease symptoms and the high oxygen requirements, in addition to the metabolic stress and the increment of the nutritional requirements caused by pneumonia associated with COVID-19. For those who were on MV, the NUTRIC score was recorded; however, this tool showed no patient with nutritional risk. This could be because this is a tool that underestimates the risk when it is used in young people, patients with just one organ failure, or those with few comorbidities (27).
Additionally, it was found that on day 14 and after patients were P-E, the percentage of infusion and the adequacy decreased by up to half. Even if that was not the purpose of this study, a possible explanation is that the first 15 days were, in our experience, when patients were still metabolically unstable, with more vasopressor requirements forcing the healthcare providers to reduce to a minimum or stop the infusion without prior notice. As reported in other publications, 20 % of our patients had P-E dysphagia and had to continue with EN (28-30). Some logistic problems could have been involved in the delay of infusion, such as the time of colocation of another NGT or collocating a long-term access as gastrostomy, resulting in a delay of up to three days, something that was probably partially solved considering that after the V-VST test, 13 % of patients were prescribed with at least glucose intravenous (IV) solutions. Strengthening communication strategies to achieve the administration of EN and fulfil full nutrient requirements in all patients is essential.
In a more detailed analysis of the EN, it was found that NNC supplies an important percentage of the total energy, with propofol being the primary source. This was also reflected in the macronutrient distribution during the reported days. During the initial days, the lipid infusion (mainly supplied by propofol) represented 70 % of the total energy until day 14, when the macronutrient proportion was better proportioned. This was similar to what Dickerson et al. reported, who evaluated different clinical trials in which patients required the infusion of propofol and nutritional support. They found that propofol infusion can provide about 356 kcal of total energy-infused, which contributes 24 % of the energy infused (31). Some studies reported an association between propofol infusion (made with soy lipids; 100 mg/ml) and triglycerides in patients with and without COVID-19 (32,33). In our study, no correlation was identified between high triglycerides levels and propofol infusion but with the total energy infusion; additionally, we found an increment of CRP with the propofol infusion. Moreover, it was found that protein intake, arginine, and n-3PUFA reduce the mortality risk.
Concerning n-3 PUFA, this reduction in the mortality risk reminds us of the importance of trying to keep the n-6:n-3 PUFA ratio low to avoid promoting an inflammatory state (34). Around 23 % of COVID-19 patients present thromboembolism complications (35) and arachidonic acid (n-6 PUFA) promotes one of the pathways for platelet activation through the production of prostaglandins type 2 and thromboxanes type 2, which contributes to the pathogenesis of thrombosis (36). The n-3 PUFA benefits are associated with the fact that this fatty acid competes for the same enzyme that metabolizes the n-6 PUFA into arachidonic acid; moreover, when n-3 PUFA is metabolized, it is a former of specialized pro-resolving mediators such as resolvins, protectins, and maresins (37). Doaei S et al. found that 1 g of n-3 PUFA supplemented in critically ill patients’ EN had a greater survival rate and better biochemical parameters and kidney function (38).
Equally important, arginine has been highly researched due to its immune properties. It works as a precursor of macrophages, and some studies have reported that supplementation could improve the response of T and Th lymphocytes. L-arginine is the substrate for nitric oxide formation, which plays a role in the improvement of endothelial activity, the same endothelial activity that has been shown to decrease in COVID-19 patients (39). One study found that plasma L-arginine levels were reduced in hospitalized COVID-19 patients compared to asymptomatic adults and that there was an inversely proportional correlation with disease severity. In an RCT where they supplemented 1.66 g L-arginine orally twice a day in patients hospitalized for severe COVID-19, it was found that supplemented patients decreased LOS and the need for MV compared to the placebo group. These results were confirmed after adjusting for possible confounding variables (40).
This work demonstrated that nutrition plays an essential role in the comprehensive management of patients and can positively impact COVID-19 patient outcomes. The inclusion of dieticians and nutrition professionals in multidisciplinary treatment teams should be contemplated. There remain areas of opportunity for improving nutritional support, and specifically, EN achieves greater effectiveness. Furthermore, this study reaffirmed that achieving at least 80 % of the energy and protein requirements, as well as n-3 PUFA and arginine supplementation, could be associated with lower mortality in COVID-19 patients.
We recognize the limitations of our study, mainly derived from the restrictions imposed on and reduction of nutrition personnel in the first months of the pandemic to avoid more contagion. As a result, the nutritional care process could not be thoroughly carried out, so several aspects were not documented in the first months of the pandemic, which can be explored in subsequent studies.