Introduction
Epilepsy affects more than 70 million people worldwide (Thijs, Surges, O'Brien & Sander, 2019). It is characterized by the predisposition to suffer seizures as well as its consequences at neurologic, cognitive, psychological, and social levels (Fisher et al., 2014). Most patients with epilepsy achieve adequate seizure control with antiepileptic drugs (AEDs). However, approximately 30% of patients continue to have seizures despite appropriate trials of two or more AEDs (Kwan, Schachter & Brodie, 2011) and suffer what is known as drug-resistant epilepsy. In most of these cases surgery may be a suitable treatment (Helmstaedter, 2013).
Despite the efficacy of both treatments in seizure control, premature mortality in people with epilepsy is estimated to be 2.6 times greater than in the general population (Levira et al., 2017). This data has been attributed to several causes such as sudden unexpected death, status epilepticus, and most frequently, epilepsy-related comorbidities. These comorbidities include psychological disturbances, mainly depression and anxiety; neurological diseases such as dementia, migraine, and cerebrovascular disease, this latter accounting for approximately 30% of newly diagnosed epilepsy in adults (Vivanco-Hidalgo et al., 2017); and non-neurological components such as heart disease or hypertension, with higher incidence of cardiovascular morbidity and mortality in people with epilepsy compared to the general population (Nair, Harikrishnan, Sarma & Thomas, 2016; Nass, Hampel, Elger & Surges, 2019; Yuen, Keezer & Sander, 2018).
Cardiovascular activity and epilepsy can interact in several ways. Firstly, in people with epilepsy, the uncontrollability and unpredictability of seizures, and the amplitude of the consequences of epilepsy affect all the aspects of the individual. The side-effects of treatments induce a long-lasting burden, making epilepsy a suitable model of chronic stress (Cano-López & González-Bono, 2019). Traditionally, chronic stress has been considered as a risk factor for cardiovascular disease in the general population (Emery, Stoney, Thayer, Williams & Bodine, 2018), and it is possible that stressful states associated with epilepsy could contribute to cardiovascular disturbances. Secondly, acute stress has been widely considered as a precipitating factor of seizures (Novakova, Harris & Reuber, 2017; Mckee & Privitera 2017; Van Campen, Jansen, de Graan, Braun & Joels, 2014) with cardiovascular variations a relevant part of the stress response. Thirdly, stress response, seizures, and epilepsy comorbidities involve key brain regions such as the hippocampus, the amygdala, and the frontal cortex (Novakova, Harris, Ponnusamy & Reuber, 2013; Elger, Johnston & Hoppe, 2017; Błaszczyk & Czuczwar, 2016). Finally, AEDs can affect electrical properties of cardiomyocytes, circulating blood components, and the activity of the autonomic nervous system (ANS) that lead to enhanced sympathetic tone (Nass et al., 2019).
Inter-ictal and ictal impairments of cardiovascular activity have been reported. Higher HR levels in the morning and lower levels at night have been reported in 22 adults with drug-resistant epilepsy in comparison with healthy controls - without relationship with self-reported stress (Novakova et al., 2017). This alteration in the HR circadian pattern, which can be interpreted as an indicator of a chronically stressed state, was not related to daily event-related stress perceptions, suggesting that simple self-reports cannot be used as a proxy of physiological arousal in patients suffering from seizures and stress (Novakova et al., 2017). During complex partial seizures, HR increases have also been found and, although non-unanimously, the hemisphere of EA seems relevant for the magnitude of the response, being especially prominent in patients with right temporal lobe epilepsy (Kirchner, Pauli, Hilz, Neundörfer & Stefan, 2002).
While the underlying mechanisms have not been totally clarified, epilepsy was not solely associated with vagal suppression as a risk factor for arrhythmias, but also with sympathetic activation, vagal activation, and sympathetic-vagal suppression (for review, Lotufo, Valiengo, Benseñor & Brunoni, 2012). Indeed, HR variability (HRV) is proposed as an interesting proxy for autonomic activity in the heart with particular interest in the high-frequency and low-frequency indexes, related to vagal and sympathetic activity, respectively (Lotufo et al., 2012).
The study of cardiovascular activity in epilepsy has been mainly restricted to epilepsy-related stimuli such as AEDs and seizures, obviating the accumulative effect of the amount and diversity of stress sources to which patients are daily exposed. The administration of other potential stressors in the laboratory could help to clarify the involved mechanisms of the cardiovascular alterations in this population. With this rationale, acute physical stress was administered to 30 patients compared with matched controls by a performance of the maximal treadmill. Patients achieved a lower peak HR than controls, but also a lower performance in the exercise (Fialho, Pagani, Walz, Wolf & Lin, 2017). In this study, female sex, age of epilepsy onset, number of secondarily generalised seizures and polytherapy were associated with lower cardiovascular fitness (Fialho et al., 2017). However, patients with epilepsy have to cope with daily cognitive challenges and, to our knowledge, no studies have been carried out with the focus on cardiovascular response to psychological/cognitive stress.
In healthy samples, changes in HR are reliably evoked by psychological stressors, and these stressor-evoked cardiovascular changes have been linked to cardiovascular disease risk (Thayer & Lane, 2009; Ginty, Kraynak, Fisher & Gianaros, 2017). There is a certain consensus that individuals who exhibit an exaggerated cardiovascular response are at elevated risk of cardiovascular disease, acute cardiovascular reactions to psychological stressors being the most heavily investigated stress-related parameters of cardiovascular risk (Ginty et al., 2017). Interestingly, it has been classically considered a right-sided predominance for the control of HR with a loss of parasympathetic control in addition to the increased sympathetic tone associated with stroke; and a left-sided predominance in the genesis of arrhythmias (Behbahani, Dabanloo, Nasrabadi & Dourado, 2016; Constantinescu et al., 2017).
With this in mind, the aim of this study was to analyse the effects of different acute stressors in the cardiovascular and psychological response in people with drug resistant epilepsy, considering the cognitive performance, the EA hemisphere, and sex. Results will provide preliminary data about cardiovascular response to a cognitive stressor and after a neuropsychological assessment in people with epilepsy in comparison with a rest condition, considering the hemisphere of EA, sex, and cognitive performance. For this, two types of stressors have been administered: the Stroop task and neuropsychological assessment. The Stroop task is frequently applied as a stress paradigm in neuropsychology to investigate the human psychophysiological response to stress (MacLeod, 1991; Renaud & Blondin, 1997), including emotional responses, increased autonomic reactivity, and HR (Delaney & Brodie, 2000). Neuropsychological assessment is frequently administered to these patients, which can be considered as a challenge to their cognitive deficits and a source of evaluative threat. The hemisphere of EA and sex will be considered as far as certain hemispheric specialisation in the regulation of cognitive and physiological demands in acute stress has been reported (Qin, Hermans, Van marle, Luo & Fernández, 2009; Porcelli et al., 2008; Czéh, Pérez-Cruz, Fuchs & Flügge, 2008; Zhang et al., 2018; Allendorfer & Szaflarski, 2014; Allendorfer et al., 2014; Mcklveen, Myers & Herman, 2015) and women have been related to lower cardiovascular fitness in response to acute physical stress (Fialho et al., 2017). Cognitive performance will be evaluated with Epitrack, a brief cognitive test that evaluates executive and attentional functioning (Lutz & Helmstaedter, 2005; Helmstaedter et al., 2010; Kadish, Baumann, Pietz, Schubert-Bast & Reuner, 2013).
Method
Participants
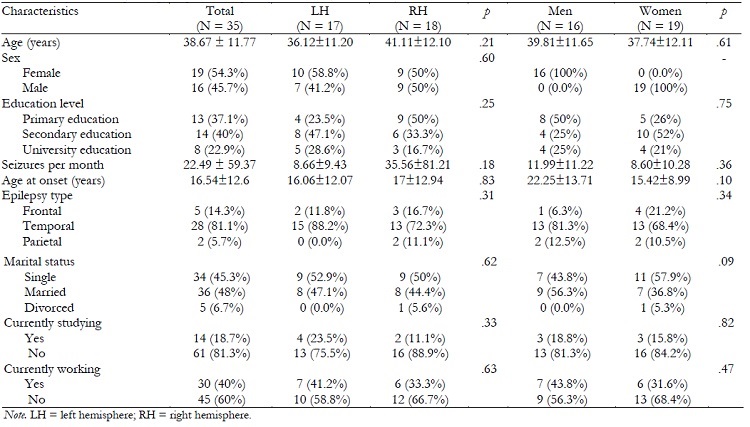
The sample was composed of 35 patients with diagnosed drug-resistant epilepsy, 17 of them (seven men and 10 women) with EA in left hemisphere (LH) and 18 (nine men and nine women) with EA in right hemisphere (RH). The age of the sample ranged from 18 to 59 years (M = 38.69, SD = 11.77), and the individuals suffered epilepsy at an average of 22.14 years (SD = 16.89). Characteristics of the sample are detailed in the Table 1.
Procedure
The procedure was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Hospital Universitario y Politécnico La Fe (Valencia, Spain). Medical history provided demographic characteristics of the patients (sex, age, and educational level) and clinical data (age at epilepsy onset, duration of epilepsy in years, frequency of seizures per month, seizure type, number and type of AEDs). Presurgical assessment was made by staff members of the multidisciplinary team and based on a comprehensive evaluation that included: seizure history and semiology; neurologic examination; long-term video-EEG monitoring; 3-Tesla magnetic resonance imaging (MRI); fluorodeoxyglucose (FDG)-positron emission tomography (PET); single photon emission computed tomography (SPECT); psychiatric assessment; and neuropsychological evaluation for all patients.
During the neuropsychological evaluation, patients signed the informed consent. Afterwards, a Polar V800 portable device was fixed to the chest of the subject at the level of the lower third of the sternum to continuously register HRV. After 10 minutes of habituation, HRV was recorded during a period of 10 minutes in rest (baseline). Mood and anxiety state questionnaires were then administered. As part of the neuropsychological assessment, a Stroop task was administered (Stroop task) being HRV registered during the entire performance of the task. A neuropsychological protocol was then applied according with the International League Against Epilepsy (ILAE) recommendations and, immediately afterwards, HRV was also registered during a period of 10 minutes (post-assessment). Finally, patients again completed mood and anxiety state questionnaires. Fifteen minutes after the Stroop task, patients underwent the Epitrack Brief Cognitive Test (EBCT). The duration of the neuropsychological evaluation lasted between 3 and 3.5 hours with the appropriate rest periods to avoid fatigue.
Variables
Cardiovascular recording
To record HR and HRV, a Polar V800 portable device consisting of a stable polyamide case with HR electrodes attached to an elastic belt was fitted to the chest of the subject. The registration and estimation of HRV by this device has been validated (Giles, Draper & Neil, 2016). HRV was recorded by the Polar system during a 10 min rest period (baseline), during the Stroop task (Stroop task), and during 10 min after the neuropsychological assessment (post-assessment), at a sampling frequency of 1000 Hz, and providing a temporal resolution of one ms for each HRV interval. Recordings were transferred to a password-protected PC under ASCII format via Polar-specific software (Polar® ProTrainer 5 software version 5.35.161). To filter and process the signal, HRV Kubios 2.2 Analysis Software was used. The five central minutes in each period (baseline, Stroop condition, and post-task) were analysed in accordance with European Society of Cardiology Task Force and the American Society of Stimulation and Electrophysiology guidelines (1996). The following parameters were calculated for the analysis of HRV: (a) time domain: HR; RR intervals; successive RR basic quadratic differences (RMSSD); number of consecutive RR intervals differing more than 50 ms (NN50); the proportion of NN50 divided by the total number of NN (pNN50); and (b) frequency domain: absolute power of low frequency bands (LF) (0.04-0.15 Hz); absolute power of high frequency bands (HF) (0.15-0.4 Hz); relative power of the low frequency bands (LF nu); relative power of high frequency bands (HF nu); and relation between the LF and HF band powers (LF/HF). Reactivities and recoveries were calculated. Reactivity is the difference between Stroop condition levels minus baseline levels and recovery is the difference between post-assessment levels minus Stroop task levels.
Mood and anxiety state
To measure mood state, the Spanish version of the positive and negative affect schedule (PANAS; Watson, Clark & Tellegen, 1988) was used. This is a self-reported questionnaire of 20 items, consisting of two subscales, one that measures positive affect and the other negative affect, each of which consists of 10 items. A five-point Likert scale was used, where one was ‘very slightly or not at all' and five ‘extremely'.
To measure state anxiety, the state trait anxiety inventory was used (STAI; Spielberger, Gorsuch, Lushene, Vagg & Jacobs, 1970). This instrument includes two subscales, the STAI-E, which evaluates the current state of anxiety; and the trait anxiety scale (STAI-R), which evaluates relatively stable aspects of anxiety. Each subscale has 20 items classified on a four-point scale, where zero was ‘not at all' and three ‘very much'.
Cognitive performance
Cognitive performance was assessed by means the numerical Stroop task and the EBCT.
The numerical Stroop task was administered employing Psychology Experiment Building Language software (PEBL; Mueller, 2012). In this task, participants were asked to indicate, as quickly and accurately as possible, how many items appeared in each trial. The number of items ranged from one to three, and participants had to press the numbers one, two, or three on the keyboard with their dominant hand depending on the number of characters that appeared on the screen. A block of 24 practice trials, and two experimental blocks were included with 84 trials each. Each block consisted of three types of stimuli: alphabetic characters (neutral condition, e.g., Z, GGG, MM); digits with values similar to the number of characters presented (congruent condition, e.g: 1, 22, 333); and digits with values that differed from the number of characters presented (incongruent condition, e.g., 2, 33, 111). The three conditions were represented in a random and proportional way in the three blocks. In each trial a cross-shaped mask was presented for 1000 ms. and, immediately thereafter, the stimulus was presented until the participant gave a response for a maximum time of 2000 ms. Finally, the number of hits and the reaction time in each condition were recorded.
The EBCT (Lutz & Helmstaedter, 2005) is a 15-min screening tool explicitly designed to track the cognitive side-effects of AEDs. It comprises of six subtests requiring attention, cognitive tracking, and working memory: an interference test, a trail-making test (parts A and B), a test of response inhibition, digit span backward, word fluency, and a maze test. A total age-adjusted Epitrack score was computed, ranged from nine to 49 points. Cutting scores for different cognitive functioning were: 39-49 for excellent cognitive performance, 32-38 for average adjusted performance, 29-31 for minor impairment, and 9-28 for severe impairment.
Statistical analysis
We used the Kolmogorov-Smirnov test to test the normality of the data. When data were not normally distributed (as correct answers of the Stroop test in congruent, incongruent, and control conditions), a logarithmic transformation was performed to normalise the variable.
To examine differences by ‘sex' or ‘hemisphere of EA' in the characteristics of the sample, the chi-square test was used for categorical variables and the t-test for independent samples for continuous variables. To investigate the effectiveness of the stressors on mood and state anxiety, univariate ANOVAs were performed, with ‘sex' and ‘hemisphere of EA' as between-subject factors, using baseline scores as covariates. To analyse the effectiveness of the stressors on cardiovascular measurements, repeated measures ANOVAs were carried out for HR, RR intervals, RMSSD, NN50, pNN50, LF, LF nu, HF, HF nu, and LF/HF, with ‘hemisphere of EA' and ‘sex' as between-subject factors, and ‘moment' as a within-subjects factor (Stroop task, and post-assessment), using baseline levels as a covariate. When a factor was significant in the previous ANOVAs, Bonferroni adjustments for the p values were performed as post hoc analysis. To examine relationships among variables, Pearson correlations were performed.
Moderator analysis using PROCESS (v2.13.6) were performed to determine potential effects of ‘sex' or ‘hemisphere of EA' in the relationships between cardiovascular activation and cognitive performance considering the cardiovascular activation as a dependent variable and cognitive performance as an independent variable.
Statistical analyses were carried out using SPSS 22.0 and two-tailed tests with p set to .05 were considered as significant.
Results
Characteristics of the sample
No significant differences were found in age, age at the epilepsy onset, or seizure frequency depending on ‘hemisphere of EA' or ‘sex'. Groups did not differ in the distribution of the frequencies in educational level, epilepsy type, or work, educational or marital status. Characteristics of the total sample and groups are detailed in the Table 1.
Cardiovascular and psychological response to stressors depending on the hemisphere of EA and sex
Significant effects of ‘moment*hemisphere of EA' and ‘sex' were found on HR (F(1, 30) = 4.18, p = .05, η 2 p = .12, and F(1, 30) = 4.65, p = .039, η 2 p = .13, respectively). Specifically, higher HR decreases were found in LH patients than in RH patients at post-assessment (p = .05). Additionally, women had higher HR than in men, independently of the moment of assessment (p = .039).
Additionally, a significant effect of ‘moment*hemisphere of EA' was found on RR interval (F(1, 30) = 4.08, p = .050, η 2 p = .12), with significant increases in the LH group (p = .01) not found in the RH patients (p = .280). No other significant effects of the ‘hemisphere of EA' were found in other cardiovascular parameters.
A significant effect of ‘moment' was found on LF in the total sample (F(1, 30) = 4.96, p = .03, η 2 p = .14), with LF decreases during the Stroop task and increases in the post-assessment condition.
A lack of significant effect of ‘hemisphere of EA', ‘sex' or their interactions were found for psychological response.
Cognitive performance depending on the hemisphere of EA and sex
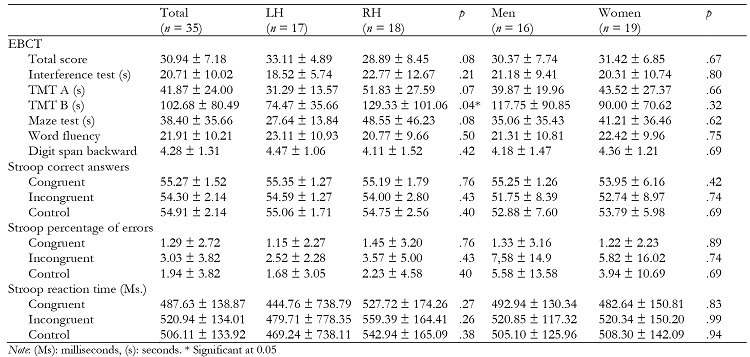
No significant effects of ‘hemisphere of EA' or ‘sex' were found on Stroop correct answers or reaction time.
In the EBCT, a significant effect of ‘hemisphere of EA' was found on TMT B (F(1, 31) = 4.36, p = .040, η 2 p = .12), with higher scores in the RH patients than in the LH group. Moreover, the TMT B subscale score was negatively related to HR activity during Stroop task and post-assessment (r(34) = -.62, p = .010 and r(34) = -.53, p = .030, respectively) in LH patients, but not in RH patients. No significant effects of ‘sex' were found.
In the total sample, HR levels during Stroop task were related to EBCT total score (r(34) = .38, p = .030), but not at post-assessment.
Descriptive of cognitive variables are found in Table 2.
Moderation role of hemisphere of EA on the relationships between cardiovascular variables and cognitive performance
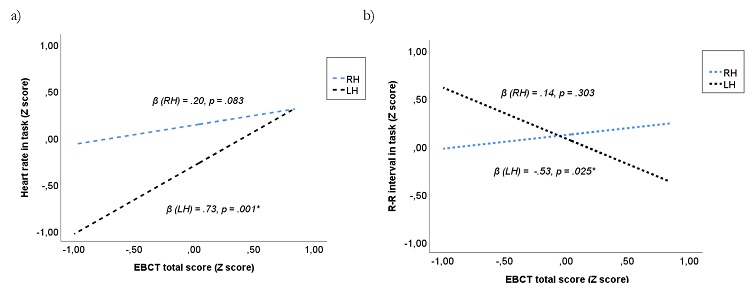
In the total sample, there was a lack of significant effects of ‘hemisphere of EA' or its interactions on cardiovascular variables at Stroop condition. To explore whether the hemisphere of EA could moderate the interaction between cardiovascular activity during the Stroop task and cognitive performance, moderation analyses were carried out. Results showed that ‘hemisphere of EA*EBCT total score' explained a higher percentage of variance of HR during the Stroop task than both factors independently (ΔR² = .04, ΔF(1, 30) = 4.98, p = .030). In fact, HR during the Stroop task was explained by the EBCT total score in the LH group (β = .73, t = 3.54, p = .001, 95% CI = .31, 1.15), but not in RH group (β = .20, t = 1.79, p = .083, 95% CI = -.02, .44). Congruently, ‘hemisphere of EA* EBCT total score' significantly explained the R-R interval during Stroop task (ΔR² = .07, ΔF(1, 30) = 7.73, p = .009), with significant relationships between total score of EBCT and R-R interval in LH group, but not in RH patients (β = -.53, t = -2.35, p = .025, 95% CI = -.99, -.07 and β = .14, t = 1.04, p = .303, 95% CI = -.13, .42, respectively). Figure 1 illustrates these moderation effects. No other moderation effects of ‘group' or ‘sex' were found.
Discussion
The aim of this study was to analyse the effects of different acute stressors in the cardiovascular response in people with drug resistant epilepsy, considering the hemisphere of EA, sex, and cognitive performance. Results show that a long-lasting neuropsychological assessment is capable of producing a hemisphere-modulated cardiovascular response, with a more pronounced HR decrease (and R-R interval increases) at post-assessment in LH patients than in the RH individuals. Additionally, HR is higher in women than men, independently of the moment of assessment. Cardiovascular response to an attentional task does not induce a sympathetic response depending on the hemisphere of EA or sex, although the hemisphere of EA moderates the relationships between cardiovascular activity and cognitive performance.
Results of the present study cannot be attributed to patient characteristics since no differences between groups were found in sociodemographic or clinical variables (i.e., age at the epilepsy onset, seizure frequency, and epilepsy type). Although no differences between the distribution of men and women in the sample were found, sex showed a significant effect on HR with significantly higher HR in women. These differences in HR were independent of the moment of assessment and they were not accompanied by differences by sex in cardiac variability. Other studies have shown lower HR in women with epilepsy than in male patients in the pre-ictal period (Behbahani, Dabanloo, Nasrabadi & Dourado, 2018) and during complex seizures (Kirchner et al., 2002).
Regarding response to stimuli, the Stroop task induced LF decreases in the total sample, and this made interpretation difficult since it reflects a mixture of sympathetic and parasympathetic activity (Thayer, Åhs, Fredrikson, Sollers & Wager, 2012). The Stroop task has been proposed as a stressor able to induce cardiovascular response in healthy samples (Bali & Jaggi, 2015). In our sample, no significant activation was found in other cardiovascular parameters. Although this is speculative, it is possible that an attentional task was not a significant challenge for patients with cognitive impairments but relatively preserved attention. Another possibility is that patients showed a blunted cardiovascular response as an indicator of disruption in adaptive processes. In fact, a diminished sympathetic response to a mental stress test has been found in multiple sclerosis patients (Vlcek et al., 2018). Moreover, this sympathetic response to stressors can be associated with activity and connectivity brain systems (Ginty et al., 2017), suggesting that miscalibration in cardiovascular activity and neural networks can be interactive. However, scarce studies on this topic makes that this rationale emerge as speculative.
However, a long-lasting neuropsychological evaluation, which includes the assessment of different cognitive domains, shows a different response depending on the hemisphere of EA. Specifically, we found higher HR decreases and greater RR increases in the LH group than in the RH group at the post-assessment period. In this sense, cardiovascular recovery after neuropsychological assessment seems to be deficient in RH patients, suggesting certain hemispheric predominance for cardiovascular activity. In contrast, LH patients showed lower HR at the post-assessment period and, therefore, a greater recovery slope. Although, to our knowledge, no studies evaluating cardiovascular response to stress in epilepsy depending on the hemisphere of EA are available, these results agree with previous studies employing other research approaches that found a hemispheric specialisation for modulation of the sympathetic nervous system with increase activation by the RH (Tegeler, Shaltout, Tegeler, Gerdes & Lee, 2015; Behbahani et al., 2016; Constantinescu et al., 2017). Although this remains speculative, our results suggest that the affectation of the RH in epilepsy patients could impact negatively on the sympathetic control in response to acute stress.
Regarding other cardiovascular parameters, we found decreases in LF during the Stroop task and increases in LF at the post-assessment period in the total sample. Contrary to our results, previous studies have found hemispheric specialisation for these cardiovascular parameters, RH activation being related to prominent tachycardia (Xavier et al., 2009) and RH anesthesia being related to HR increases and LF/HF ratio and HF decreases (Ahern et al., 2001). Moreover, other studies have found highly elevated parasympathetic activity in the left TLE with hippocampal sclerosis compared to right TLE with hippocampal sclerosis (Ghchime et al., 2016). All studies mentioned above empathising a RH lateralisation of sympathetic cardiac control and LH lateralisation of parasympathetic control (Behbahani et al., 2016; Oppenheimer, 2006; Saleh et al., 2000; Xavier et al., 2009).
In the present study, sympathetic activity was related to cognitive performance. Specifically, HR levels were related to total EBCT score, indicating that cognitive performance is relevant to study sympathetic activation. Moreover, these results depended on the side of seizure focus, since TMT B subscale score was negatively related to HR in the Stroop task and post-assessment periods only in LH patients. Additionally, we found a moderation effect of side of seizure focus on the relation between EBCT total score and HR only during the Stroop task period.
In LH patients, HR and R-R interval depended on EBCT performance. Although there are no studies that have evaluated cardiovascular response to stress in people with epilepsy, previous studies investigating stress response in patients with epilepsy found a bilateral medial frontal/cingulate and superior frontal gyri activation in response to acute stress in LH patients (Allendofer et al., 2014), as well as frontal asymmetry in activation response to stress, with right frontal activation during an acute stressor (Zhang et al., 2018). These results suggest the relevance of prefrontal and executive function in modulation of HR and stress response, as well as an HR specialisation (Mcklveen et al., 2015; Remue et al., 2016; Sullivan & Gratton, 1999).
Some limitations can be reported in the present study. Firstly, patients were treated with AEDs polytherapy, and the AEDs combination was individualised for each patient, and this may affect the results differently. In fact, previous studies found that certain AEDs such as valproate, phenytoin, and carbamazepine could favour cardiovascular disease as side-effects (Nair et al., 2016; Vivanco-Hidalgo et al., 2017). Secondly, there are important methodological differences between our study and previous works in the study of cardiovascular response, since other groups have used the Wada test, resting HR, direct activation of cortical zone or ictal HRV signals. Thirdly, although all the patients presented drug-resistant epilepsy, the group is heterogeneous in terms of the exact localisation of EA. Although 80% of patients presented TLE, results should be interpreting with caution. Fourthly, we have not used a perceived stress questionnaire after the Stroop task, which would provide more information about the general stress response. Finally, for future research, other biological variables (such as cortisol or glucose) should be considered, since they could provide more information about the stress response in these patients.
In conclusion, our preliminary data suggest a different pattern of cardiovascular response to stress in patients with epilepsy depending on the hemisphere of EA, as well as a moderating role of the hemisphere of EA on the relationship between cardiovascular response and cognitive performance. Our results could have clinical implications from a preventive perspective, since results suggest that the hemisphere of EA may be a relevant factor for coping with stress in people with refractory epilepsy and targeting of future treatment programmes. Moreover, there are no studies that have evaluated cardiovascular response to acute stress in patients with epilepsy, so this study provides information for future research in this sample.