Introduction
Individuals suffering from specific phobia usually respond with a feeling of fear accompanied by a variety of behavioural, physiological, endocrine and neural changes when they are exposed to their feared object. However, some types of phobias, such as blood-injury-injection phobia (BII), differ from other specific phobias in the reactions provoked by the phobic stimulus both at a peripheral and central level (Ayala, Meuret, & Ritz, 2010; Sánchez-Navarro et al., 2018; Sarlo, Buodo, Devigili, Munafò, & Palomba, 2011).
Several early and late components of the event-related potentials (ERPs) have been found to be influenced by emotional stimuli in both phobic and healthy participants. Research conducted on non-phobia participants has revealed that P200 is enhanced by affective pictures, probably reflecting processes related to automatic, exogenous attention (e.g., Carretié, Mercado, Tapia & Hinojosa, 2001; Carretié, Hinojosa, Martín-Loeches, Mercado,& Tapia, 2004), while P300 has been related to the motivational significance of the affective stimuli and might reflect the allocation of processing resources (e.g., attention) to motivationally relevant stimuli (Hajcak, Weinberg, MacNamara, & Foti, 2012; Palomba, Angrilli, & Mini, 1997). In this line, it has been found that unpleasant affective pictures capture greater attention than other affective pictures, as reflected by P200 (Carretié et al., 2004), while highly arousing affective pictures provoke larger late positive potential (LPP) amplitudes than non-affective, neutral pictures (e.g., Bradley, Hamby, Löw, & Lang, 2007; Schupp et al., 2000).
Brain activity in specific phobia, e.g., animal phobia, is usually characterised by enhanced amplitude of short- and long-latency ERPs. Short-latency ERPs, such as P100 and N170 components are enhanced in spider phobia when subjects are presented with their phobic stimuli (Michalowski, Melzig, Weike, Stockburger, Schupp, & Hamm, 2009; Michalowski, Pané-Farré, Löw, & Hamm, 2015) and in some cases also when viewing other non-feared stimuli (Kolassa, Musial, Mohr, Trippe, & Miltner, 2005; Michalowski et al., 2015). This finding has been interpreted as indicative of both an early facilitation of the encoding of fear stimuli and a state of increased unspecific vigilance provoked by highly emotional pictures, which may promote the generalization of fear to other sources.
Previous studies have also shown an increased amplitude of late ERPs in the time range of 300-700 ms, corresponding to P300 and late positive potential (LPP), a positive long-latency wave that appears when the participant is presented with visual affective stimuli. These late ERPs show larger amplitudes mainly at parietal sites, when phobic participants are presented with the object of their phobia (Kolassa et al., 2005; Michalowski et al., 2009; Miltner et al., 2005; Schienle, Schäfer, & Naumann, 2008). These ERPs have been interpreted as indicators of the emotional and motivational significance of the fear-relevant stimuli and of a greater processing activity. Increased amplitude of short- and long-latency ERPs gives support to the idea that phobic individuals allocate more attentional resources towards fear-relevant stimuli, thus showing an attentional bias to any possible source of danger (Michalowski et al., 2015). This attentional bias is, in addition, a common feature of anxiety states and anxiety disorders (MacLeod & Mathews, 2012).
In contrast to animal phobia, few works have been conducted to study the central nervous system activity related to blood phobia. It has been reported that the passive viewing of pictures depicting blood-related contents (e.g., injuries and mutilations) promotes a rapid identification and processing of the stimuli in non-phobic participants, and leads to the engagement of the motivational defence system (e.g., Bradley, Codispoti, Cuthbert, & Lang, 2001; Bradley et al., 2007). Accordingly, these pictures demand greater attention resources in comparison to other highly arousing, unpleasant stimuli (e.g., attack) or neutral contents (Schupp, Junghöffer, Weike, & Hamm, 2004; Sarlo, Buodo, Poli, & Palomba, 2005). This increased allocation of processing resources to blood-related stimuli leads to increased amplitudes of short- and long-latency ERPs. In BII-fearful participants, Buodo, Sarlo, & Munafò (2010) found enhanced amplitude of N2pc (a short-latency wave, appearing at 180-240 ms, related to spatial allocation of attention) to pictures depicting injuries. Related results have been reported by Leutgeb, Schwab, Scharfmüller, Höfler, & Schienle (2018), who found an enhancement P100 to blood stimuli presented without context information. In addition, a larger activity at 190-250 ms at parieto-occipital locations has been reported in BII-fearful participants in comparison to healthy participants provoked by unpleasant stimuli (both disorder-relevant and non-disorder relevant), which could be related to a generalized cortical response to unpleasant stimuli (Buodo, Peyk, Junghöfer, Palomba, & Rockstroh, 2007). Thus, the increased amplitude of short-latency ERPs in BII-fearful participants seems to be not exclusive to the reaction to blood-related stimuli, but rather a generalized response to unpleasant stimuli.
Similar contrasting data have been reported regarding the late ERPs elicited by the passive viewing of phobic stimuli in BII phobia subjects. A repeated finding is that BII-fearful individuals do not show an enhancement of late ERPs during the passive viewing of their feared object. For example, Buodo, Sarlo, Codispoti, & Palomba (2006) find no enhancement of the LPP in BII phobia participants exposed to disorder-relevant materials. This result has been interpreted as the lack of an attentional bias to threat-relevant information, which may indicate that fewer processing resources are allocated to fear-related stimuli in subjects suffering from BII phobia. This reduced assignment of processing resources to blood-related stimuli has been interpreted as a cognitive avoidance strategy to reduce fear in the presence or in anticipation of the feared stimuli (Buodo et al., 2010). This idea has, however, been challenged by Leutgeb, Schäfer, & Schienle (2011), who reported enhanced LPP (300-700 ms) in parietal sites in individuals suffering from dental phobia (a specific phobia included in the subtype of BII phobia) provoked by pictures related to their fear in comparison with control subjects. The authors interpreted their results as an increased motivated attention to disorder-relevant stimuli. These seemingly contradictory results in short- and long-latency ERPs and their relationship with attention and motivational processes in BII phobia require further research.
The present experiment
The automatic allocation of processing resources to fear-related stimuli might be influenced by threat cues that anticipate the content of the upcoming stimulus and, therefore, increase the subject's reaction. A threat cue is supposed to sensitize sensory encoding and increase selective attention to danger stimuli (Bublatzky & Schupp, 2012). It is not clear, however, whether a threat cue will increase selective attention to phobic-related stimuli or promote a lack of attentional bias in BII phobia. For this purpose, we decided to incorporate a cue that anticipates the presentation of threat and non-threat stimuli in blood phobia. It has been found in animal phobias, e.g., spider phobia, that a threat cue preceding the presentation of the feared stimulus increases short-latency ERPs, P1 and early posterior negativity at 200-300 ms, as well as LPP (Michalowski et al., 2015). Following the findings of Buodo et al. (2007), and with the idea that there is of a lack of attentional bias in BII individuals, we expected that a cue signalling the upcoming phobic picture would increase the amplitude of short-latency ERPs to blood-related pictures, while no effects were expected on long-latency waves (e.g., P300).
In addition to BII phobia participants, we selected individuals suffering from another specific phobia (snake phobia), as a control phobic group, together with a non-phobia control group. Following previous research on animal phobias, threat cues were expected to induce short and long latency ERPs of larger amplitudes to snake-related pictures in snake phobia participants than in healthy individuals.
Material and Methods
Participants
The Spanish version of the Mutilation Questionnaire (MQ) and the Snake Questionnaire (SNAQ) (Klorman, Hastings, Weerts, Melamed, & Lang, 1974) were administered to 312 undergraduate students. Participants were preliminarily included in the high blood-fear group (n = 21) if their scores in the MQ were above the 90th percentile (> 16) of the scoring distribution of the whole sample, and their SNAQ scores were below the 90th percentile (< 16). High snake-fear subjects (n = 19) were preliminarily selected if their SNAQ scores were above the 90th percentile (> 16) of the scoring distribution of the whole sample, and their MQ scores were below the 90th percentile (< 16). Then, participants were screened by means of a semi-structured interview by a clinical psychologist in order to check whether they fulfilled the DSM-V criteria for specific phobia (American Psychiatric Association, 2013). The phobic samples comprised 13 participants (mean age = 20.38, SD = 2.10, range = 18-26) that matched the criteria for BII phobia -but not those for snake phobia - and 12 participants (mean age =19.17, SD = 1.53, range = 18-23), which matched the criteria for snake phobia-but not for BII phobia. The BII fearful group showed a mean MQ score of 20.77 (SD = 3.09, range = 17-28), and a mean SNAQ score of 7.69 (SD = 3.42, range = 3-13). The snake fearful group showed a mean SNAQ score of 20.17 (SD = 2.25, range = 17-24), and a mean MQ score of 8.84 (SD = 2.52, range = 6-13). A group of non-phobia participants was also randomly selected from those individuals whose MQ and SNAQ scorings were below the 75th percentile (< 11 for both questionnaires). The control group comprised 14 participants (mean age = 19.86, SD = 1.17, range = 19-22), with a mean MQ score of 6.21 (SD = 2.39, range = 3-10), and a mean SPQ score of 6.07 (SD = 2.50, range = 2-10).
All participants under study signed a written informed consent, and all procedures were conducted in accordance with the Declaration of Helsinki and the Ethics Committee of the University of Murcia.
Materials and design
We selected 45 affective pictures1 from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008). Pictures comprised 3 specific contents (15 pictures per content): blood-injection-injury, snakes, and neutral/household objects. The 3 categories of pictures differed in affective valence, F(2,4 2) = 137.22, p < .0001 (blood-related < snakes < neutral). Blood-related pictures were more unpleasant than snake and neutral pictures and, in turn, snake pictures were more unpleasant than neutral pictures (all Ps < .0001). Arousal also differed between the 3 picture categories, F(2, 42) = 285.93, p < .0001. Blood-related and snake pictures did not differ in arousal level (p = .374), and both sets of pictures were higher in arousal than neutral pictures (both Ps < .0001).
The task consisted of an S1-S2 paradigm, in which S1, the cue, was a word presented for 1000 ms signalling the content of the picture (S2) that would appear 3000 ms after S1 onset (see Figure 1). The three words selected to match picture content were the Spanish words for “blood”, “snake”, and “neutral”. Each picture (S2) was presented for 2000 ms.
We constructed 90 trials, with each picture appearing twice along the task, once in each half of the task. The 45 pictures were randomly distributed in each half of the task. The intertrial intervals varied randomly from 7 to 11 s. We constructed 3 different presentation sequences and each subject was randomly assigned to one of them.
Data recording and processing
The electroencephalogram (EEG) was continuously recorded from 28 active electrodes (Acticap, Brain Products, Germany), using a BrainAmp amplifier (Brain Products, Germany), according to the international 10-20 system (Fp1, Fp2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, C3, Cz, C4, T7, T8, CP5, CP1, CP2, CP6, TP10, P7, P3, Pz, P4, P8, O1, O2). The electrode impedances were kept below 5 kΩ and electrodes were referenced on-line to the left earlobe. Horizontal (outer canthi of both eyes) and vertical (below the right eye) electrooculograms (EOG) were also recorded. The sampling rate was of 500 Hz, and the band-pass filter was set at 0.5-40 Hz. The EEG analysis was performed off-line using the EEGLAB software (Delorme & Makeig, 2004) and ERPLAB software (Lopez-Calderon & Luck, 2014). The EEG recording was re-referenced to linked earlobes and frequencies below 0.5 Hz and 30 Hz were filtered out. We used an automatic-removal method (Artifact Subspace Reconstruction; Chang, Hsu, Pion-Tonachini, & Jung, 2018) to identify and remove channels containing irreparable artifacts, and the rejected channels were interpolated. The EEG was epoched into 5300 ms intervals -covering from 300 ms before S1 until S2 offset- and base-line corrected using the average of activity in the 200 ms previous to S1 onset. The epochs were then corrected for ocular artifacts using a regressive algorithm (Gratton, Coles, & Donchin, 1983) on the bipolar transformation of the EOG electrodes (for superior EOG we used the average of Fp1 and Fp2). Epochs containing artifacts exceeding (100 (V in any channel were then excluded from further analysis. The percentage of trials accepted for analyses was 97.71 (SD = 5) for blood-related trials, 96.63 (SD = 7.44) for snake-related trials, and 96.47 (SD = 6.99) for neutral-related trials (the percentage of valid trials did not statistically differ between groups, F(2, 35) = .337, p > .05, type of trial, F(2, 70) = 2.48, p > .05, or the interaction between group and type of trial, F(4, 70) = .88, p > .05). The EEG epochs were then averaged for each subject and picture category.
Following previous research (e.g., Sarlo et al., 2011; Schupp et al., 2000), we extracted the parameters of interest from 9 locations: F3, Fz, F4, C3, Cz, C4, P3, Pz, and P4. The local peak amplitude elicited by S2 was computed for P200 as the most positive peak over central electrodes in a time window between 150-250 ms (Bublatzky & Schupp, 2013). P300 was computed over parietal locations as the most positive peak between 300-500 ms after S2 (Buodo et al., 2006; Bublatzky & Schupp, 2013). All the parameters obtained were deviated from a baseline defined as the mean in the 200 ms interval preceding S2 onset.
Procedure
Participants were accommodated in an armchair located 0.75 m in front of a computer screen and all sensors were attached. Participants were then informed that a word would appear on the screen indicating the type of picture that would follow some seconds later. They were required to attend to both the words and the pictures.
After the cued viewing-task all the electrodes were removed. Participants were then asked to view each picture again in a free viewing time setting and to rate the affective valence and arousal of each picture using computerized scales. The software employed for picture display presented, separately and after each picture, a 9-point rating scale for each dimension, with 9 indicating a high rating (i.e., high pleasure, high arousal), and 1 a low rating (i.e., low pleasure, low arousal).
Data analysis
For each ERP, we conducted a mixed model ANOVA, 2 (Group: BII phobia, snake phobia, and control) X 3 (Content: blood-related, snakes, neutral) X 3 (Laterality: left, midline, right), with Group as between-subjects factor, and Picture category and Laterality as within-subjects variables.
For each subjective variable, we conducted a mixed model ANOVA, 2 (Group) X 3 (Content), with Group as between-subjects factor, and Content as within-subject variable.
All the statistical analyses were performed with the PASW package (version 19; Chicago, IL). A measure of the effect size, partial eta-squared (ηp 2), was obtained for the main statistical tests. When appropriate, we applied a Greenhouse-Geisser adjustment to the degrees of freedom in repeated measures tests to correct any potential inflation of the reported probability values, and epsilon values (ε) are reported (Bagiella, Sloan, & Heitjan, 2000). Post-hoc comparisons were performed with a Bonferroni correction to control the overall level of significance (Keselman, 1998).
Results
P200
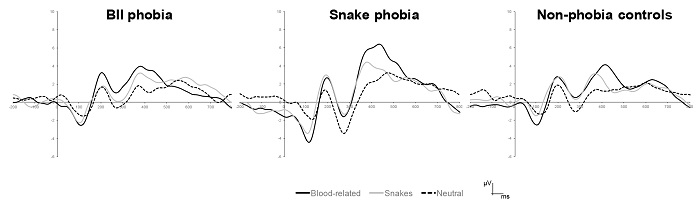
We found a significant main effect of Content, F(2, 70) = 22.03, p < .0001, ηp 2 = .39. Paired comparisons revealed that blood-related pictures (Mean = 5.17, SD = 2.67) and snake pictures (Mean = 4.48, SD = 2.85) provoked larger peak amplitudes than neutral pictures (Mean = 2.69, SD = 2.72) (p < .0001 for both comparisons). However, this effect was qualified by a significant Group X Content interaction, F(4, 70) = 3.95, p < .006, ηp 2 = .18 (see Figure 2). In the BII phobia group, the Content did not reach statistical significance, F(2, 22) = 3.19, p > .05. In contrast, we found a significant effect of Content in the snake phobia group, F(2, 22) = 25.55, p < .0001, ηp 2 = .70, with both blood-related pictures and snake pictures provoking larger peak amplitudes than neutral pictures (p < .001 for both comparisons). In the control group, the Content factor also reached statistical significance, F(2, 26) = 6.09, p < .01, ηp 2 = .32, and paired comparisons only revealed that blood-related pictures provoked larger peak amplitudes than neutral pictures (p < .01).
A significant main effect of Laterality was also found, F(2, 70) = 7.92, p < .01, ε = .75, ηp 2 = .19. Follow-up analyses showed that peak amplitude at midline (Mean = 4.57, SD = 2.70) was larger than amplitudes at either left (Mean = 3.84, SD = 2.18) or right electrodes (Mean = 3.94, SD = 2.38) (p < .05 for both comparisons).
P300
A significant main effect of Content was found, F(2, 70) = 39.59, p < .0001, ηp 2 = .53 (see Figure 3). Paired comparisons showed that both blood-related pictures (Mean = 9.46, SD = 3.47) and snake pictures (Mean = 8.96, SD = 3.71) provoked similar peak amplitudes and both were larger than the amplitudes provoked by the neutral pictures (Mean = 5.13, SD = 3.21) (p < .0001 for both comparisons). This effect was qualified by a Content X Laterality significant interaction, F(4, 140) = 3.37, p < .05, ηp 2 = .09. Follow-up analyses revealed that amplitudes were larger in midline locations than in left or right electrodes for blood-related pictures (p < .05 for both comparisons) and snake pictures (p < .0001 for both comparisons). Regarding neutral pictures, midline amplitude was larger than the peak amplitude at right location (p < .01).

Figure 3 ERPs provoked by the pictures (average for all participants) recorded at parietal electrodes.
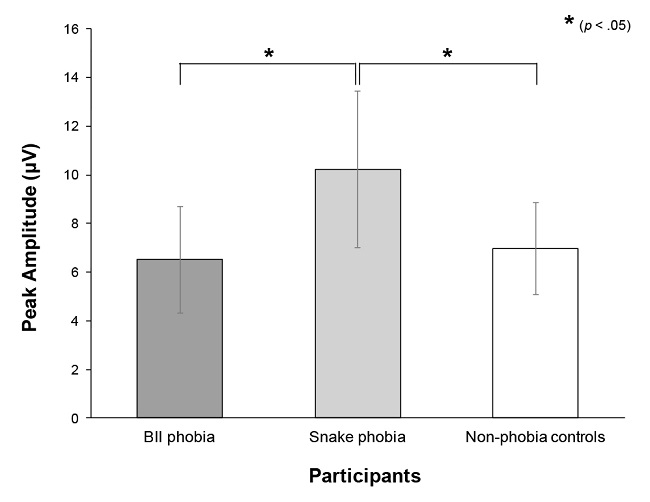
A significant main effect of Group was found, F(2, 35) = 8.31, p < .001, ηp 2 = .32 (see Figure 4). Paired comparisons revealed that snake phobia participants (Mean = 10.23, SD = 3.22) showed larger peak amplitudes than both BII phobia (Mean = 6.51, SD = 2.18) and control participants (Mean = 6.95, SD = 1.89) (p < .01 for both comparisons). A main effect of laterality was also found, F(2, 70) = 17.34, p < .0001, ε = .84, ηp 2 = .33. Paired comparisons showed that peak amplitude at midline electrode (Mean = 8.32, SD = 3.40) was larger than amplitudes at either left (Mean = 7.56, SD = 2.61) or right electrodes (Mean = 7.67, SD = 2.83) (p < .0001 for both comparisons). However, a significant interaction between Group and Laterality, F(4, 70) = 3.54, p < .05, ηp 2 = .17, revealed that this effect only appeared in snake phobia participants (p < .01 for comparisons between midline and either left or right locations).
Subjective ratings
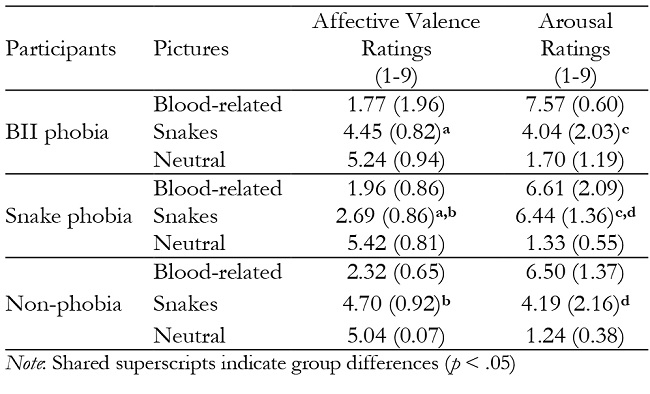
Affective valence. We found a significant main effect of the Content, F(2, 70) = 264.55, p < .0001, ηp 2 = .88. Blood-related pictures were rated with the lowest affective valence ratings (Mean = 2.03, SD = 0.69), followed by snake pictures (Mean = 3.98, SD = 1.23) and, in turn, by neutral pictures (Mean = 5.22, SD = 0.69) (p < .0001 for all comparisons). This effect was qualified by a Group X Content interaction, F(4, 70) = 15.95, p < .0001, ηp 2 = .48 (see Table 1). Follow-up analyses showed that blood-phobia participants and control participants rated blood-related pictures with the lowest affective valence (p < .0001), whereas snake and neutral pictures were rated with similar values (p > .05). Snake phobia participants rated blood-related pictures with the lowest affective valence, followed by snake pictures and, in turn, by neutral pictures (p < .05 for all comparisons). The comparison between groups in each picture content only revealed differences for snake pictures, F(2, 35) = 19.69, p < .0001, with snake phobia individuals rating snake pictures with lower affective valence than both BII phobia and control participants (p < .0001).
A significant main effect of Group was also found, F(2, 35) = 4.73, p < .05 , ηp 2 = .21, with paired comparisons showing that snake phobia participants rated all the pictures with lower affective valence than control participants (p < .05).
Arousal. A significant main effect of Content, F(2, 70) = 193.1, p < .0001 , ηp 2 = .85, revealed that blood-related pictures (Mean = 6.87, SD = 1.51) were rated with the highest arousal, followed by snake pictures (Mean = 4.85, SD = 2.14) and, in turn, by neutral pictures (Mean = 1.41, SD = 0.78) (p < .0001 for all comparisons). This effect was qualified by a Group X Content interaction, F(4, 70) = 6.87, p < .0001, ηp 2 = .28. BII phobia participants and control participants rated blood-related pictures with the highest arousal ratings, followed by snake pictures and, in turn, by neutral pictures (p < .01 for all comparisons; see Table 1). Snake phobia individuals, however, rated blood-related pictures and snake pictures with similar arousal values, and both were higher than the arousal ratings of the neutral pictures (p < .0001 for both comparisons). Additional comparisons between groups for each picture content only showed statistical differences for snake pictures, F(2, 35) = 6.14, p < .01, revealing that snake phobia participants rated the snake pictures with higher arousal ratings than both BII phobia and control participants (p < .05 for both comparisons).
Discussion
The aim of this work was to investigate the cortical responses promoted by phobia-related pictures following threat and non-threat cues in BII phobia, snake phobia, and non-phobic participants. For comparison purposes with previous research we limit the discussion to the early (P200) and late components (P300) of the evoked potentials elicited by fear and neutral pictures.
Overall, fear stimuli enhanced P200 and P300 amplitudes. However, in the BII phobia group P200 did not differentiate between picture contents and, therefore, did not show an increase provoked by blood-related stimuli in comparison with the snake and the neutral pictures, in spite of the fact that BII participants rated the blood-related pictures as the more unpleasant and arousing. Early ERPs are related to automatic exogenous attention and are modulated by the emotional salience of the stimuli (Carretié, 2014). For example, unpleasant pictures usually provoke larger P200 amplitudes than pleasant pictures (e.g., Carretié et al., 2001; Delplanque, Lavoie, Hot, Silvert, & Sequeira, 2004; Olofsson & Polich, 2007). Some research has also shown an enhancement of early ERPs in blood phobia exposed to blood-related stimuli in comparison with other contents (Buodo et al., 2007; Leutgeb et al., 2018). The discrepancy between the data of these studies and ours might result from the cue signalling the upcoming picture used in our experiment. In this regard, our results might be associated with a blunted reaction to the blood-related pictures resulting from the anticipation of the feared stimuli in BII phobia individuals. The threat cue could have induced anticipatory reactions aimed at avoiding the feared stimulus and reducing its potential aversive effects. This reaction could manifest itself as a low engagement of early automatic attention resources towards the phobia-relevant picture, as evidenced by P200.
A possible explanation to the absence of an increase in P200 amplitude provoked by blood-related pictures in BII phobia participants is the activation of central mechanisms of emotion regulation triggered by the threat cue. Some research has reported an increase in the activity of brain regions related to emotion regulation (e.g., ventral prefrontal cortex) in BII phobia individuals when exposed to blood-related pictures, which could lead to the inhibition of several autonomic responses such as heart rate or blood pressure (e.g., Caseras et al., 2010; Thayer & Brosschot, 2005; Thayer & Lane, 2000). According to this interpretation, the threat cue would provoke an overregulation of emotion in the BII phobia participants, resulting in a decrease of the attentional resources assigned to the phobic object, as revealed by the reduced P200 amplitude. In this regard, an increase in alpha power to mutilation no-go trials in BII phobia participants in a go/no-go task has also been reported, and has been interpreted as a passive avoidance of the phobic object (Mennella et al., 2017). Our results support these findings by showing that signalling the phobic object to BII phobia individuals provokes a passive avoidance state with a reduction in attentional resources.
This idea is supported by the comparison between the responses of the snake phobia and those of the control group participants. Snake phobia participants showed the greatest P200 amplitude to both snake and blood-related pictures, thus showing the expected response in individuals suffering from animal phobia as described in the literature (e.g., Kolassa et al., 2005; Michalowski et al., 2015). These larger amplitudes could be related to the greater automatic attention elicited by both type of pictures in comparison to the P200 amplitude elicited by the neutral pictures, as well as to an increase in the vigilance of threatening stimuli, whether or not they are the feared objects. Control participants showed larger P200 amplitudes to blood-related pictures than to neutral pictures in accordance with the greater automatic attention elicited by the most arousing, blood-related pictures, as previously reported (e.g., Carretié et al., 2001). The high arousing properties of the blood-related stimuli, as evidenced by the subjective ratings of the pictures, might explain the results in both groups. Paralleling the P200 results, snake phobia participants rated their disorder-relevant pictures with similar arousal than blood-related pictures. In the same vein, the control group rated the blood-related pictures with the greatest arousal and unpleasantness, and these pictures provoked the largest P200 responses in this group. This result is in agreement with previous findings reporting that blood-related, injury and mutilation pictures are among the most threatening stimuli, provoking a rapid automatic identification and processing, as well as the engagement of the motivational defence system (e.g., Azevedo et al., 2005; Bradley et al., 2001, 2007). For example, when compared to other arousing affective pictures, blood-related pictures provoke greater autonomic (e.g., SCRs) and somatic (corrugator supercilii activity) responses (e.g., Bradley et al., 2001; Sánchez-Navarro, Martínez-Selva, & Román, 2006; Sánchez-Navarro et al., 2012b), larger ERPs and electrocortical activity (e.g., Sarlo et al., 2005; Schupp et al., 2004), greater brain activity within the posterior ventral cortex (e.g., Bradley et al., 2003), as well as higher enzymatic and hormonal activity (Codispoti et al., 2003; Sánchez-Navarro, Maldonado, Martínez-Selva, Enguix, & Ortiz, 2012a).
Our results also revealed that disorder-relevant pictures (both blood-related and snakes) promoted higher P300 amplitudes than neutral pictures in all groups. This ERP has been proposed as an index of endogenous attention and memory storage (Olofsson, Nordin, Sequeira, & Polich, 2008). In the context of emotion, it has also been related to the allocation of processing resources to arousing as well as motivationally significant stimuli (Amrhein, Mühlberger, Pauli, & Wiedemann, 2004; Cano, Class, & Polich, 2009; Ferrari, Bradley, Codispoti & Lang, 2010; Olofsson et al., 2008). Our data from the BII phobia group are in agreement with previous findings (e.g., Buodo et al., 2007; Leutgeb et al., 2018) since blood-related pictures evoked larger P300 amplitudes than neutral stimuli and similar to those elicited by the snake pictures. These results could give support to the idea of a lack of the attentional bias to threat-relevant information in BII phobia as revealed by the P300. Alternatively, since the same ERP pattern was obtained in snake phobia individuals, it could be postulated that the preceding cue alerting about the upcoming fearing picture has prepared the individuals for the subsequent picture by triggering mechanisms of emotional control that preclude the attentional bias to their disorder-relevant stimulus usually found in these phobias (e.g., Leutgeb et al., 2009; Scharmüller, Leutgeb, Schäfer, Köchel, & Schienle, 2011; Schienle et al., 2008). The absence of an experimental condition in which pictures are not preceded by a cue restricts, however, this interpretation, and it remains an open question for further research. In any case, the larger P300 amplitudes found in the snake phobia group, in comparison to BII phobia and control participants, point to a hypervigilant state in the animal phobia group, which constitutes a typical response of anxious states (Buodo et al., 2007).
In conclusion, our data revealed that a cue signalling a disorder-relevant stimulus reduced automatic attention towards the upcoming phobic stimulus in BII phobia participants, as revealed by P200 amplitude, in comparison to snake phobia and non-phobia participants. This result supports the hypothesis of a deficient allocation of attentional resources to phobic-related pictures in BII fearful participants, in contrast to snake phobia. According to previous research, these anomalous responses can be related to an overregulation of emotion in BII phobia individuals when confronted with their feared object. This hypothesis addresses cognitive and emotional mechanisms in BII phobia that might be of clinical relevance and needs to be confirmed by future research.
Footnotes.- 1 IAPS code for the pictures used in the study. Blood-related pictures: 3030, 3051, 3053, 3060, 3064, 3071, 3102, 3150, 3170, 3250, 3301, 3400, 3550, 9405, 9594. Snake pictures: 1019, 1026, 1030, 1040, 1050, 1051, 1052, 1070, 1080, 1090, 1101, 1110, 1113, 1114, 1120. Neutral pictures: 7004, 7006, 7009, 7010, 7020, 7025, 7035, 7040, 7080, 7175, 7185, 7187, 7217, 7233, 7235.