Introduction
tDCS is a non-invasive brain stimulation (NIBS) technique able to modulate neural excitability using a weak electric current (usually ranging from 1 to 2 mA) between two (or more) electrodes of different polarity on the scalp (Utz et al., 2010). Its neuromodulatory effect is related to changes in neuronal polarization, changing spontaneous fire rates (Purpura & McMurty, 1965). Generally speaking, anodal stimulation induces membrane depolarization resulting in an increase of neural excitability, while cathodal stimulation induces membrane hyperpolarization resulting in decreases of neural excitability (Nitsche & Paulus, 2000; Paulus, 2011). However, cortical modulation does not depend on stimulation polarity alone, but also on neuronal type and orientation and on the intensity of stimulation (Purpura & McMurty, 1965). Besides, electric stimulation can also produce changes in the excitability of neural populations distant from the locus of stimulation that are functionally related to the stimulated areas (Bortoletto et al., 2015).
The effects induced by tDCS can last for some time after the end of the stimulation (Nitsche et al., 2003). These after-effects depend on the duration and intensity of the applied electric current (Utz et al., 2010), may have a duration on the order of hours and days (see Pasow et al., 2017), and seem to be related to plastic changes in the synapses (similar to long-term potentiation -LTP- and depression
-LTD-) dependent of NMDA receptors (Liebetanz et al., 2002; Nitsche et al., 2003; Nitsche et al., 2004). However, it should be noted that it is still not clear how putative LTP/LTD-like effects induced by NIBS correspond to the changes in brain activity or connectivity observed using functional neuroimaging techniques (Bartrés-Faz & Vidal-Piñeiro, 2016).
Long-term memory refers to information stored for a significant time period, from minutes to years. Usually, it can be divided in two main categories: declarative memory and procedural memory (Smith & Kosslyn, 2008), depending on whether the access to stored information is conscious or not, respectively. Within declarative memory, Tulving (1972) distinguished between episodic and semantic memory, defining the former as the conscious knowledge of temporally dated events and of the spatiotemporal relations among those events. This type of memory seems to be quite vulnerable to information transformation and loss due to neural disfunction than other memory systems (Tulving, 1972, 1983), including normal aging and Alzheimer's Disease (see Tromp et al., 2015 for a review).
Prefrontal cortex (PFC) is an essential structure for information integration and executive control, with an important role in brain networks that control the different types of memory (Reynolds et al., 2006), including both long-term and short-term memory processes (Gazzaniga et al., 2009). The long-term memory functional system includes three processes: information encoding, consolidation and retrieval (Breedlove et al., 2010), and PFC would be involved in all the three processes along with other cortical and subcortical structures (Smith & Kosslyn, 2008; Gazzaniga et al., 2009). In particular, PFC seems to be involved in a brain network for episodic memory that also includes medial temporal lobe and parietal cortex (Rugg & Wilding, 2000).
According to the HERA model (Hemispheric encoding/retrieval asymmetry) there is a hemispheric asymmetry in frontal lobe activation: left prefrontal regions would be more active during the encoding of new verbal information in episodic memory, while right prefrontal regions would be more active during the information retrieval (Habib et al., 2003).
In the last fifteen years, several studies about the possible applications of the tDCS to improve cognitive function in healthy population as well as with cognitive decline have been conducted, especially regarding memory. For example, it has been consistently proven that anodal stimulation on F3, over the left dorsolateral PFC (DLPFC), improves performance in verbal working memory tasks, with more correct responses (Andrews et al., 2011; Berryhill & Jones, 2012; Fregni et al., 2005) and/or reduced reaction times (Hoy et al., 2013; Mulquiney et al., 2011).
Most of the studies about the potential efficacy of tDCS in healthy population to improve episodic memory have targeted frontal locations (see Galli et al., 2019 for a comprehensive review and meta-analysis). In general, those studies that applied anodal tDCS over the DLPFC found a facilitation effect on episodic memory in verbal (Floel et al., 2008; Gaynor & Chua, 2017; Habich et al., 2017; Hammer et al., 2011; Javadi et al., 2012; Javadi & Walsh, 2012; Javadi & Cheng, 2013; Lafontaine et al., 2013; Manenti et al., 2013; Nikolin et al., 2015; Sandrini et al., 2016) and non verbal (Balzarotti & Colombo, 2016; Morgan et al., 2014) tasks, or in tasks that included verbal and non verbal items (Gray et al., 2015; Leach et al., 2018). On the other hand, it seems that cathodal stimulation on the DLPFC leads to a deterioration in short-term learning (Elmer et al., 2009).
Despite this, there are also some contradictory results in previous studies: some of those that used anodal stimulation over the DLPFC failed to find any effects (de Lara et al., 2017; Leshikar et al., 2017; Smirni et al., 2015) or found poorer accuracy than in the sham condition (Manuel & Schnider., 2016; Marián et al., 2018; Zwissler et al., 2014); and improvements in episodic memory were found using cathodal DLPFC stimulation (Smirni et al., 2015).
The mentioned studies applied different stimulation protocols (i. e. intensity, duration, electrode dimensions…) and memory tasks, which may explain the heterogeneity in the results. Besides, the stimulation was not always applied during the same memory process: while most of the previous studies stimulated participants' DLPFC during the encoding process (e. g. Zwissler et al., 2014), others applied the stimulation during the consolidation period (e. g. Javadi & Cheng, 2013; Smirni et al., 2015), others during the retrieval stage (e. g. Javadi & Walsh, 2012), while others applied the tDCS “offline” (before the memory task) (e.g. Lafontaine et al., 2013; Lu et al., 2015).
The main aim of this study was to evaluate the effects of anodal tDCS on episodic memory in a group of healthy young participants. For this purpose, the effects of anodal tDCS (applied over the left DLPFC during the encoding phase of an episodic memory task) on the accuracy rate and reaction time of immediate and delayed recall phases of the task were studied. Our working hypothesis was that 2 mA anodal tDCS applied over the left DLPFC during 18 minutes per session in two consecutive days would enhance immediate and delayed memory recall in healthy young participants.
A second aim of this study was to evaluate the effects of this type of stimulation on the scores obtained in the neuropsychological evaluation that the participants underwent one month before and one week after the stimulation sessions.
Finally, we also aimed to validate the experimental protocol (stimulation with anodal tDCS over the left DLPFC during the encoding phase of an episodic memory task) of the present study, with intent to use it to improve episodic memory in samples of healthy elderly or with mild cognitive impairment participants.
Method
Participants
Thirty participants (7 men and 23 women; mean age 21.7 years) from an initial sample of 35 college students were selected. Participants were healthy, with normal or corrected vision, right handed (assessed with Edinburgh Handedness Inventory, Oldfield 1971), and between 18 and 28 years old. Exclusion criteria were: obtaining a score out of ± 1 standard deviation range from the group average in two tests of the pre-intervention neuropsychological battery, having a family history of epileptic attacks, suffering from any neurologic or psychiatric disorder, consume of psychiatric drugs or any psychoactive substance, pregnancy, and metallic implants in the head.
The 30 participants were assigned to the experimental or control group using random pairing attending gender, age, and neuropsychological test scores. Both groups showed no significant differences in those variables. Throughout the study, 7 subjects missed sessions or refused to continue, thus the final sample was 23 subjects (12 in the experimental group, 11 in the control group), these groups also showed no significant differences between them in gender, age or neuropsychological test scores.
All participants gave written informed consent prior to the experiment and were reimbursed with 25 euros (in school supplies), for their participation. The study was approved by the Galician Clinical Research Ethical Committee, and complies with the Helsinki Declaration.
Experimental procedure
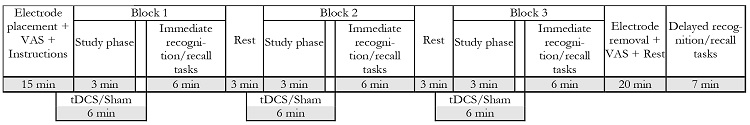
Participants attended 5 experimental sessions. On two of them (second and third) they received real or sham tDCS (Figure 1).
In the first session, participants received the information needed about the study and signed an informed consent form. After that, the pre-intervention neuropsychological assessment battery was applied. Based on its results we proceeded to select the participants that would be part of the study. This session lasted for one hour and took place a month before the intervention sessions.
The two intervention sessions (second and third experimental sessions) took place in consecutive days and lasted for 80 minutes. Participants were asked at the beginning and end of each session to fill a Visual Analogue Scale (VAS) to assess any possible discomfort during the stimulation. After placing the anodal and cathodal electrodes at F3 and Fp2 respectively, participants were seated in front of a computer screen, placed at 60 cm from them, where they were prompted to read about the structure of the session and the task's instructions. Stimulation (real vs sham tDCS) was administered beginning 2 minutes before the study phase of the memory task until 1 minute after its end, for a total of 6 minutes of stimulation per task block, resulting in a total of 18 minutes of stimulation per session (Figure 2).
After the stimulation finished, the instructions to the immediate recognition/recall tasks (old - new recognition and place recall) were presented, and the participants were asked to complete them. This procedure was identical for each of the 3 blocks. Once the participants had finished the 3 blocks of the task, the stimulation electrodes were removed and asked them to fill another VAS. Twenty minutes after finalizing the third block, participants were asked to perform the delayed recognition/recall tasks.
The fourth experimental session took place one day after the third session and lasted for 10 minutes. In this session participants were asked to complete the delayed recognition/recall tasks.
Finally, the fifth and last session took place a week after the third session and lasted for one hour. In this session participants were asked to complete again the delayed recognition/recall tasks and following that, the post- intervention neuropsychological assessment was carried out.

Figure 1 Time sequence of sessions. First session: Information and pre-intervention neuropsychological assessment; second and third: tDCS intervention and memory tasks; fourth: 24-hours delayed memory recall task; fifth: One-week delayed memory recall task and post-intervention neuropsychological assessment.
Neuropsychological assessment battery pre- and post-intervention
A neuropsychological battery was administered in the first session. The battery was composed by standardized neuropsychological tests in order to select the sample and compare the effects of intervention (comparison pre/post intervention). The tests were administered in the following order: Rey auditory verbal learning test (RAVLT); trail making test (B); WAIS IV subtests: digit span, arithmetic, symbol search and coding; and, lastly, a word fluency test (p/m/r).
These tests were selected in order to systematically assess the participants' long- and short-term memory, learning and attention. We deemed this necessary due to those cognitive processes being involved in the task that the participants had to perform during the intervention.
Transcranial direct current stimulation (tDCS)
Stimulation was applied in two different sessions (second and third), with a 24-hour interval between them. In our double-blind procedure, participants were randomly assigned to receive active or sham stimulation, administered by a researcher who did not know which subjects were receiving real or sham stimulation.
Stimulation was administered with BrainStim (EMS) devices, with two rubber electrodes inside cellulose saline soaked sponges. We used a 35 cm2 sponge as the anode and a 70 cm2 sponge as the cathode. The anodal electrode was sited over the left dorsolateral prefrontal cortex (F3 position, following the 10/20 IS) and the cathode over the right orbitofrontal cortex (Fp2 position).
The experimental group received a direct current of 2 mA (current density at the anode: .057 mA/cm2) in three blocks of 6 minutes each, for a total of 18 minutes per session. Stimulation was ramped up at the beginning of each block for 30 seconds until reaching 2 mA, and ramped down at the end of each block for 30 seconds. Sham stimulation was ramped up and down for 25 seconds until reaching 1.5 mA at the beginning and at the end of each block, and then stimulation ceased.
Memory tasks
In each of the two stimulation sessions, we used a declarative memory task comprising three consecutive blocks each one comprising a study phase followed 1 minute later by an immediate recognition/recall task. In addition, 20 minutes after the third block, a delayed recognition/recall test was presented (Figures 1, 2). Reaction times and accuracy rate (quantified by percentage of correct responses) in the immediate and delayed recall tests were measured.
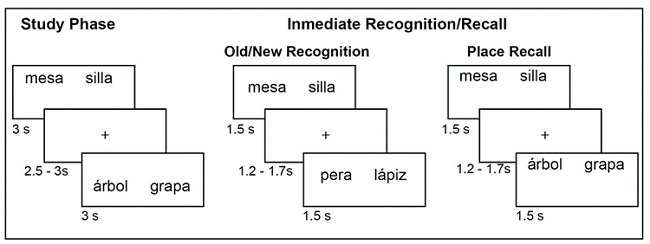
In the study phase a sequence of 30 word pairs was presented. Each word pair was presented for 3 seconds and the inter-stimulus interval was randomized to 2.5 - 3 seconds. Participants had to decide whether the words in a given word pair were semantically related or not and press one of two keyboard keys accordingly; 50% of the word pairs were semantically related and 50% had no semantic relation. These word pairs could appear at the top or bottom of the screen
The immediate and delayed recognition/recall tasks comprised two different tasks: an old-new recognition task followed by a place recall task. In the old-new recognition task a series of 60 word pairs was randomly presented in the center of the screen, and the participant had to decide by pressing one of two keys in a keyboard whether a given pair is “old” (was previously presented in the study phase) or “new”. 50% of pairs were old and 50% were new. In the place recognition task, the 30 word pairs of the study phase were randomly presented two times in the series, either at the top or bottom of the screen, and the participant had to decide if a word pair was in the same position as in the study phase or not, and press a key accordingly. In both tasks participants had a time limit of 1.5 seconds to give the response, and the inter-stimulus interval was randomized between 1.2 - 1.7 seconds (Figure 3).
One day and one week after the second intervention session, the participants had to complete the delayed recognition/recall tasks again. In the last session, the post- intervention neuropsychological assessment was carried out.
Data analysis
In order to evaluate the effects of experimental manipulation on the dependent variables (reaction time -RT- and accuracy rate (percentage of hits) -AR-) in the word pair place recall task, several repeated measures analysis of variance (ANOVA) were performed, including in all of them Group, with two levels (experimental an control), as between- subjects factor.
Firstly, the group differences in the immediate place recall task for each intervention session were analyzed. Thus, repeated measures ANOVA with factors Group and Block (3 levels) were conducted comparing the AR and RT between the 3 blocks of the immediate recall task for each intervention session.
Following this, the differences between groups in intra-session and inter-session learning were analyzed, comparing the AR and RT for immediate recall between the first and last block of each session and the first block of the first session with the last block of the second session. To this end a repeated measures ANOVA with factors Group and Session (2 levels) was conducted.
Regarding the delayed recall tasks, the differences between groups in delayed intersession learning were analyzed, comparing the AR and RT for delayed recall tasks using factors Group and Delay (4 levels). Complementarily, we compared the data of the third block of the first intervention session with the data of the intra-session delayed task, as well as the third block of the second intervention session with the other 3 delayed tasks (intra-session, 24 hour and 1 week delay). To this end, repeated measures ANOVAs with factors Group and Task (2 levels for each ANOVA) were conducted.
Finally, t-tests for dependent samples were used to compare the scores of the pre- and post- intervention neuropsychological tests, for each group; followed by a t-test for independent samples to compare post-intervention scores in both groups.
The Greenhouse-Geisser correction for the degrees of freedom was performed when the condition of sphericity was not met. In these cases, the corresponding degrees of freedom were provided. When the ANOVAs revealed significant effects due to the main factors and/or their interactions, post hoc comparisons were performed applying the Bonferroni correction. For all statistical analyses we considered a significance alpha level of < .05.
Statistical analyses were carried out with SPSS (Version 21). Researchers did not know which group received real or sham stimulation when conducting the analysis.
Results
Data analysis showed a significant main effect for within - session learning, between - session learning, and delayed learning in both AR and RT. Two significant Group x Task interactions for delayed learning were found. However, a significant Group main effect was not obtained in any of the dependent variables.
Immediate recall task
The repeated-measures ANOVA (Block x Group) showed a significant main effect for the factor Block, with an improvement of AR from the first to the third block, both in the first [F(2, 30) = 63.10; p < .001] and second [F(2, 32) = 13.88; p < 0.001] intervention sessions, and a shortening of RT in both the first [F(2, 42) = 15.57; p < .001] and second [F(1,3 0) = 6.24; p < .001] sessions (Table 1 & Figure 4A).
Post-hoc analysis showed both groups significantly increased their AR from the first to the third block in the first session (p < .001). For the second intervention session, the experimental group significantly increased their AR between blocks 1 and 3 (p = .013) and blocks 2 and 3 (p = .010), while the control group showed a progressive increase from block 1 to 3 (p = .020). Regarding RT, both groups also exhibit a significant shortening of RT in the first session from block 1 to 3 (p = .007 for the experimental group, and p = .044 for the control group) and between block 1 and 2 for the experimental group (p = .001). In the second intervention session both groups showed again a significant decrease of RT from block 1 to 3 (p = .031).
In order to determine the global effect of immediate learning, repeated measures ANOVA (Session x Group) were conducted to compare the AR and RT scores between the first block of the first intervention session and the third block of the second intervention session. A significant main effect for the Block factor in both AR [F(1, 21) = 299.06; p < .001], and RT [F(1, 21) = 98.75; p < .001] was observed. Post-hoc analysis showed a significant shortening of RT (p < .001) and increase of AR (p < .001) between the two blocks for both groups. The ANOVA did not show significant Group effect or interactions between factors.
Delayed recall task
Repeated measures ANOVA (Delay x Group) were conducted to compare the AR and RT obtained in each one of the four delayed place recall tasks (first intervention session delayed task, second intervention session delayed task, 24-hours delayed task and 1 week delayed task). The ANOVAs showed a significant main effect of the Delay factor for both AR [F(2, 37) = 15.89; p < .001] and RT [F(2, 44) = 13.19; p < .001], but not of the Group factor (Table 1).
Post-hoc analysis showed a significant increase in AR for both groups between the delayed recall task of the first and second intervention sessions (p = .001), and between the delayed task of the first intervention session and the delayed task 24 hours after the second intervention session (p < .001). Furthermore, both groups exhibited a significant decrease in AR between the delayed tasks 24 hours and 1 week after (p = .025). The opposite pattern is observed for RT, with a significant decrease between the delayed tasks of the first and second intervention sessions (p = .003) and the delayed task of the second intervention session and the delayed task 24 hours after (p = .001); and an increase in RT between the delayed tasks 24 hours and 1 week after (p = .011).
The experimental group displayed a significant decrease of RT between the delayed tasks of the intervention sessions (p = .009), and between the delayed task of the first intervention session and the delayed tasks 24 hours (p = .001) and 1 week after (p = .010); meanwhile, the control group showed a significant increase in RT from the delayed task 24 hours after to the delayed task 1 week after (p = .036).
To assess the effect of consolidation and learning decay due to the delay period between the immediate and delayed tasks, four repeated-measures ANOVA (Delay x Group) were conducted analyzing the last immediate (third block) place recall task of each intervention session and their corresponding 20 minutes delayed recall task. Moreover, we also used the second session last immediate recall task to compare with the delayed 24 hours and 1 week after recall tasks.
The first ANOVA (Delay x Group) compared the behavioral scores (AR and RT) between the third block of the immediate place recall task and the delayed recall task, in the first intervention session, for both groups. It revealed a significant main effect of the Delay factor both for AR [F(1, 21) = 7.19; p = .014], with a significant decrease of AR for the control group (p = .027); and a significant main effect of Delay for RT [F(1, 21) = 18.63; p < .001] with a significant decrease of RT in both groups (experimental: p = .004; control: p = .009). Again, significant Delay x Group interactions or differences between groups were not found.
The second ANOVA (Delay x Group) compared AR and RT between the third block of the immediate place recall task and the delayed recall task, in the second intervention session, for both groups. This ANOVA did not yield any significant effect or interaction.
The third ANOVA (Delay x Group) compared AR and TR between the third block of the immediate place recall task in the second intervention session and in the delayed place recall task 24 hours after, for both groups. The analysis revealed a significant interaction Delay x Group for RT [F(1, 21) = 4.32; p = .05]. Post-hoc analysis showed a decrease of RT between these two tasks, only for the experimental group (p = .01) (Figure 4B).
Finally, the fourth ANOVA (Delay x Group) analyzed AR and RT between the third block of the immediate place recall task in the second intervention session and the delayed place recall task one week after. In this analysis we also found a significant interaction Delay x Group for RT [F (1, 21) = 5.07; p = .035] between these tasks. Post- hoc analyses showed that only the control group exhibited a significant increase of RT between these two tasks (p = .018) (Figure 4B).
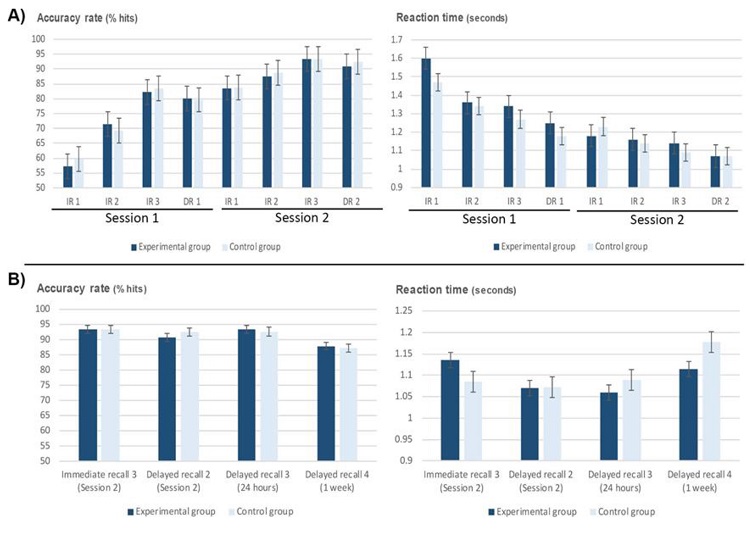
Figure 4 A) Mean values and standard error in the immediate and delayed place recall tasks during the intervention sessions for both groups. B) Mean values and standard error for the last immediate place recall task and delayed place recall tasks 1 day and 1 week after. IR = immediate recall, DR = delayed recall.
Neuropsychological assessment battery
To compare the performance of each group before and after the intervention, t-test for dependent samples for each group were conducted. The results showed a significant improvement in the post-intervention scores compared with the pre-intervention scores in both groups.
The experimental group showed a significant enhancement in RAVLT, in WAIS IV digit span (total: t = - 3.23, p = .008) and processing speed index (t = -4.25, p = .001) (Table 2). The control group showed a significant enhancement in RAVLT (recognition: t = -6.4, p < .001; total: t = -6.34, p < .001), trail making test (t = 3.77, p = .004), WAIS IV digit span (total: t = -5.12, p < .001; direct: t = -2.32, p = .042; and backwards: t = -3.63, p = .005), working memory index (t = -3.39, p = .007), symbol search (t = -3.53, p = .005), and processing speed index (t = -3.59, p = .005) (Table 2).
To compare the post-intervention scores of the neuropsychological tests between groups, t-test for independent samples were conducted. The results did not show significant differences in any of the tests of verbal memory & learning, working memory, processing speed, nor in the Trail Making Test (B). Only a significantly higher score was observed for the control group than for the experimental group in the Word Fluency Test (t = 2.942; p = .008).
Discussion
The main aim of the present study was to determine the effects of anodal tDCS, administered over the DLPFC during the encoding phase of an episodic memory task, on the accuracy rate (AR; percentage of hits) and reaction time (RT) measured in the immediate and delayed recall phases of the episodic memory task, in a group of healthy young participants.
Regarding performance in the episodic memory task, the results evidenced significant main effects for factors Block, Session, and Delay on the accuracy rate and on reaction time, but not for the Group factor. Consequently, the results did not support the initial hypothesis, since experimental (anodal tDCS) and control (sham tDCS) groups did not show significant differences in AR or RT scores of the immediate or delayed recall tasks. These results suggest that the anodal tDCS over the left DLPFC did not affect the encoding and consolidation of information in long-term memory of the young adult sample.
Even if there are several studies that found tDCS effects on episodic memory when stimulating the DLPFC in young participants (as reviewed in the introduction section), our results are in line with other studies that also failed to find tDCS effects in episodic memory paradigms (de Lara et al., 2017; Leshikar et al., 2017; Smirni et al., 2015). More importantly, our results are in line with the main finding of a recent metaanalysis, that observed that although most of the studies included showed tDCS effects on episodic memory, the metaanalysis only showed non-significant close-to-zero effects (Galli et al., 2019).
Our results showed a ceiling effect due to learning in the immediate and the second and third delayed recognition tasks in both groups (reaching around 95% of hits), so this may have made impossible to observe any further improvement due to tDCS. Besides, we used a complex paradigm that required the stimulation to be administered in blocks. Hence, the experimental procedure complexity may also have had a role in the lack of significant differences between real and sham stimulation. However, there are studies focused on tDCS effects on episodic memory in healthy individuals administering stimulation in blocks, finding improvements in the stimulated groups (Javadi & Walsh, 2012; Marshall et al., 2004), while other that applied tDCS continuously also failed to find any group differences in young participants (Cespón et al., 2017; de Lara et al., 2017; Leshikar et al., 2017; Smirni et al., 2015). Variability in the experimental protocols is so large that is really difficult to compare their results and guess which variables may be responsible for the contradictory outcomes.
In general, studies should use a large number of participants that guarantee sufficient power, and ensure that participants are sufficiently engaged and motivated (Berryhill et al., 2014). Galli et al. (2019) found that stimulating for 20 minutes or more was more effective that stimulating less than 20 minutes, and that the type of the task used to probe memory moderated the effectiveness of anodal tDCS in young participants, being recall tasks more effective than recognition tasks. The state of the stimulated brain region at the time of tDCS application, the difficulty of the task and the dosage of the stimulation are also known to influence the results (Miniussi et al., 2013; Fertonani & Miniussi, 2017).
In future tDCS studies with young populations, these variables should also be assessed, and they should include well-reasoned hypothesis (vs. exploratory studies) in order to establish more effective stimulation protocols in this type of population (Galli et al., 2019). On the other hand, despite studies regarding elderly participants are not as many as those with young participants, it seems that tDCS effects may be stronger for the former (Berryhill et al., 2014; Cespón et al., 2017), and even stronger for clinical populations (Brunoni & Vanderhasselt, 2014). This is encouraging for future studies focusing in clinical intervention.
Although the present results did not show Group effects, a significant Delay x Group interaction on RT showed that both groups presented a different evolution along the different delayed recall sessions. Hence, while the experimental group significantly decreased their RT from the third immediate recall task of the second stimulation session to the 24-hour delayed task, and kept that scores one week later; the control group did not show significant changes in RT between the third immediate recall task and the 24-hour delayed recall task and, moreover, significantly increased RT from the 24-hour delayed task to the 1-week delayed task.
However, it is necessary to point out that when comparing the reaction times between groups in each delay task, the differences did not reach significance. These results might indicate a subtle effect of anodal tDCS on memory decay due to the delay intervals, as participants who received anodal tDCS showed shorter RT 24 hours later, and maintained it one week later, while those who received sham tDCS did not show the 24-hour RT shortening and even showed a RT increase one week later. This possible modulatory effect of anodal tDCS in memory decay along time should be replicated in future studies.
The results evidenced significant main effects for factors Block, Session, and Delay on the accuracy rate and on reaction time, which reveals an improvement of performance in subjects' learning throughout the different tasks and sessions both for the immediate and delayed recognition tasks.
Regarding the immediate recall tasks we found a significant improvement in both accuracy rate (increase of the percentage of hits) and reaction time (shortening of RT) within the tasks in each session, as well as between sessions; specially from the first block of the first intervention session to the third block of the second intervention session, in which a ceiling effect for AR was achieved. For the delayed recall tasks, a progressive improvement was observed until the 1-day-later tasks (RT achieved the shortest scores in the 20-minutes delayed recall task of the second intervention session, maintaining them until the 1-day-later tasks), while in the 1-week-later task the AR decreased and the RT increased when compared to the 1-day-later task. These results support that young subjects benefited from additional practice in the tasks, as indicated by improved performance independently of anodal or sham tDCS, and constitute a learning curve of the experimental protocol.
With regard to the possible effects of intervention on the neuropsychological tests scores, significant differences between the pre- and post- intervention comparisons were obtained within each group in working memory and processing speed indexes, among other variables. These results can be attributed to the use and training of these processes throughout the study, as well as the learning of the neuropsychological tests, rather than a result of the administered stimulation. Furthermore, the comparison of post-intervention neuropsychological tests scores between groups did not show significant differences caused by anodal vs sham tDCS, except for the word fluency test, in which the control group showed better performance than the experimental group. We cannot rule out the presence of outliers in the control group that might explain this result since a non-significant trend mirroring this result was present in the pre- intervention scores.
In conclusion, robust intra-session and inter-session learning effects but no anodal tDCS effect in episodic memory performance or in pre-post neuropsychological assessment tests were found in a healthy young group. However, a subtle modulatory effect of tDCS on memory decay along delay intervals was observed, as participants who received anodal tDCS showed shorter RT 24 hours later, and maintained it one week later, while those who received sham tDCS did not show the 24 hours RT shortening and even showed a RT increase one week later.
Furthermore, the protocol used to evaluate pre- and post-intervention various cognitive domains (neuropsychological battery), and the episodic memory task used to evaluate within-session and between-session performance, showed their potential utility to be used in samples of healthy elderly or mild cognitive impairment participants. Finally, with regard to the characteristics of stimulation with tDCS to be used in future studies, the conclusions and recommendations of recent meta-analysis and reviews should be taken into account with the objective of developing more effective experimental protocols that allow for a better understanding of tDCS effects across studies.