My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
The European Journal of Psychiatry
Print version ISSN 0213-6163
Eur. J. Psychiat. vol.28 n.2 Zaragoza Apr./Jun. 2014
https://dx.doi.org/10.4321/S0213-61632014000200005
Deficit of perineuronal oligodendrocytes in the inferior parietal lobule is associated with lack of insight in schizophrenia
Victor M. Vostrikov; Natalya S. Kolomeets and Natalya A. Uranova
Laboratory of Clinical Neuropathology, Mental Health Research Center, Moscow, Russia
This study was supported by the Stanley Medical Research Institute. Grant number: 07R-1787.
ABSTRACT
Background and Objectives: Previously we reported a significant reduction in the numerical density of oligodendrocytes and oligodendrocyte clusters in the inferior parietal lobule (IPL) in schizophrenia that was associated with lack of insight. We also found a significant decrease in the number of perineuronal oligodendrocytes (PnOl) in the prefrontal cortex in schizophrenia and therefore we hypothesized that there may also be a deficit of PnOl in the IPL in schizophrenia and that it could be associated with poor insight.
Methods: We estimated the number of PnOl adjacent to pyramidal neurons in layer 3 of BA39 and BA40 in Nissl stained sections from 24 males with schizophrenia and 24 normal male controls from the Stanley Parietal Collection. The schizophrenia group was divided into three subgroups based on level of insight: poor, fair or good.
Results: We found a significant deficit of PnOl in layer 3 of BA39 and BA40 in the schizophrenia group as compared to the control group (p<0.01). In the control group but not in the schizophrenia group in BA39 the number of PnOl was significantly higher in the left hemisphere compared to the right hemisphere. In schizophrenia, in BA39 the number of PnOl was decreased in the subgroup with poor insight vs. controls. In BA40 the subgroups with both poor and fair insight were decreased vs. controls (p<0.01). In BA40 the subjects with fair insight also differed from those with good insight (p<0.01).
Conclusions: The reduction of PnOl in the IPL in schizophrenia is associated with impaired insight and lack of hemispheric asymmetry.
Keywords: Schizophrenia; Perineuronal oligodendrocytes; Inferior parietal lobule; Insight; Morphometry.
Introduction
Neuroimaging, genetic and postmortem studies have all provided evidence that a dysfunction and deficits of oligodendrocytes may be involved in further abnormalities of neuronal connectivity in schizophrenia1,2 and may also contribute to the various clinical symptoms of schizophrenia including impaired insight into their disorder3-7. Impaired insight is a core feature of schizophrenia and an important predictor of functional outcome, prognosis, and treatment adherence8. Impaired insight is associated with dysfunction of specific brain structures, including the prefrontal cortex (PFC) as well as the IPL in particular9,10. Poor insight is associated with deficient prefrontal cognitive functioning, lower prefrontal volumes and reduced gray matter volume in the IPL in schizophrenia patients10. Previously we reported a significant 15% reduction in the numerical density of oligodendrocytes6 and 25% reduction in oligodendrocyte clusters7 in the IPL in schizophrenia and the association of these measures with the lack of insight.
The IPL is among the most highly lateralized areas of the brain, and both decreased cerebral lateralization and reversed asymmetry of the IPL have been reported in schizophrenia10-13. A significant lateralization of oligodendrocyte density was detected in layer 3 of the IPL (BA39) (L>R) in the control group but not in the schizophrenia group6.
The IPL and the PFC have extensive interconnections and common cortical and subcortical target regions14. Previously we reported a reduction in the number of PnOl in layer 3 of the dorsolateral PFC in schizophrenia15. Based on these findings, we hypothesized that a deficit of PnOl may also occur in the IPL in schizophrenia, and that it may be more pronounced in the left hemisphere and in the schizophrenia subjects with poor insight as compared to those with good insight.
The aims of the study were: 1) to estimate the number of PnOl in layer 3 of the IPL (BA39, BA40) in schizophrenia and normal controls; 2) to analyze hemispheric effect on the number of PnOl and 3) to study the effect of diagnosis and insight on the number of PnOl.
Materials and Methods
Samples
Human brain specimens were donated by the Stanley Medical Research Institute's 'Parietal Collection'. The samples consisted of 48 male subjects (24 controls and 24 with schizophrenia). Diagnosis was made according to DSM-IV criteria. A postmortem estimate of each person's awareness of illness (insight) based on the person's clinical records has been previously reported6. The mean age at the time of death was 44.3 ± 9.3 years for the control group and 39.8 ± 10.7 years for the schizophrenia group. The average postmortem interval was 24.4 ± 10.8 hours for the control group and 29.1 ± 11.6 hours for the schizophrenia group. Fourteen cases were from the left hemisphere and ten cases were from the right hemisphere. Tissue of the IPL was available from one hemisphere of each brain. Complete demographic and clinical data were reported in the previous paper6.
The brain specimens were coded, and all cytoarchitectural assessments were done blindly. The angular gyrus (BA39) and the supramarginal gyrus (BA40) were identified according to macroscopic landmarks16. Ten serial sections through the IPL (every 17th section) were mounted on slides and Nissl-stained.
Morphometric analysis
The sublayers of layer 3 (a, b and c) in BA39 and BA40 were easily identified based on cytoarchitectural features. The number of PnOl was counted in each sublayer of layer 3 in BA39 and BA40. PnO1 were defined as all oligodendrocytes located within <5 μm of pyramidal neurons. Pyramidal neurons were identified by their triangular shape, oval shaped clear nucleus with a prominent nucleolus, presence of intensively stained cytoplasm and vertical apical dendrite. Oligodendrocytes were identified by the presence of a small round or oval nucleus, with relatively dense nuclear staining (more chromophilic than astroglial nuclei) and a narrow unstained rim of cytoplasm. One hundred pyramidal neurons with identifiable nucleolus were systematically randomly sampled for each sublayer. The number of pyramidal cells sampled from each subject was 300, from each group 7200, total 14,400. The number of PnOl was expressed as the number of oligodendrocytes per neuron.
Statistical analyses
Statistical analysis was performed using Statistica 7. The data were examined using the Kolmogorov-Smirnov test for normality. A Pearson correlation analysis was performed to assess possible correlations between the parameters measured and age, postmortem interval, pH, refrigerator interval, brain weight, total lifetime antipsychotics, age at onset and duration of disease. Comparisons between patients with schizophrenia and controls were performed using two-way ANOVA with the number of PnOl in three sublayers of layer 3 as the dependent variables, and diagnosis and hemispheres as the independent variables. A one-way ANOVA followed by post hoc Duncan's test was used to compare the control group and the three insight schizophrenia subgroups. A correlation analysis was also performed to assess possible correlations between the number of PnOl and the numerical density of oligodendrocyte clusters as estimated previously in the same sections7.
Results
Effects of disease and hemispheres
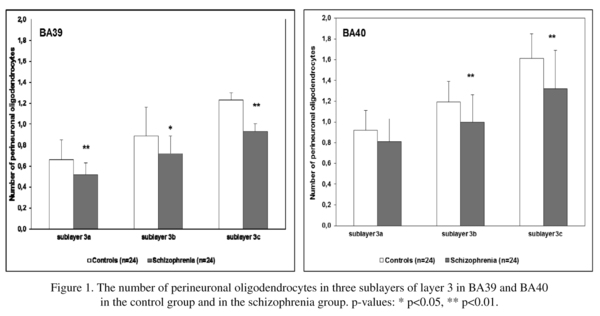
The mean numbers of PnOl and the results of their comparisons between the schizophrenia and the control groups for each sublayer of layer 3 are shown in Fig. 1. The ANOVA revealed a significant effect of diagnosis on the number of PnOl in all sublayers in BA39 [F(3,44) = 4.57, p = 0.007] and in all sublayers in BA40 [F(3,44) = 3.71, p = 0.018]. A decrease in the mean values of the parameter was found in BA39 in sublayer 3a (-21%, p = 0.004), in sublayer 3b (-20%, p = 0.013), in sublayer 3c (-24%, p = 0.004) and in BA40 in sublayer 3a (-13%, p = 0.059), in sublayer 3b (-16%, p = 0.007) and in sublayer 3c (-18%, p = 0.003) in the schizophrenia group as compared to the control group. Correlation analysis did not reveal significant effects of postmortem interval, refrigerator interval, brain weight, brain pH, age at onset or duration of disease on the number of PnOl in BA 39 and BA 40.
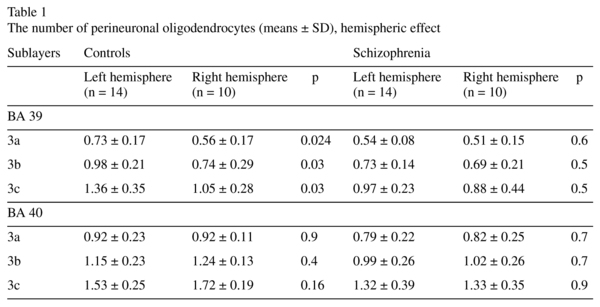
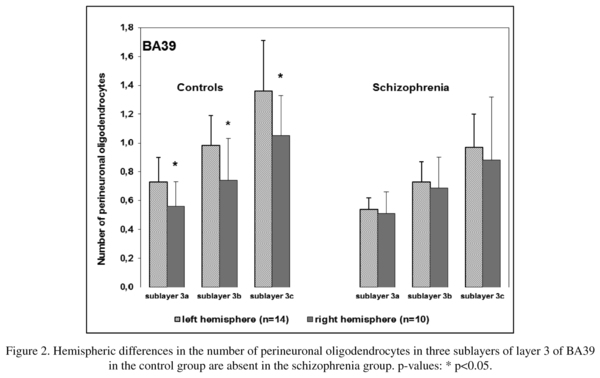
There was a significant hemispheric effect on the number of PnOl in BA39 in layer 3 [F(3,43) = 2.89, p = 0.046]. A post hoc test demonstrated a significant hemispheric difference in the control group but not in the schizophrenia group: the number of PnOl was significantly higher in the left hemisphere as compared to the right hemisphere in 3a (23%), 3b (25%) and in 3c (23%). There were no significant hemispheric differences in BA 40 (Table 1, Fig. 2).
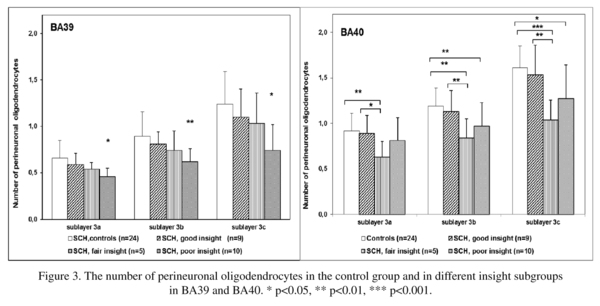
Effects of insight
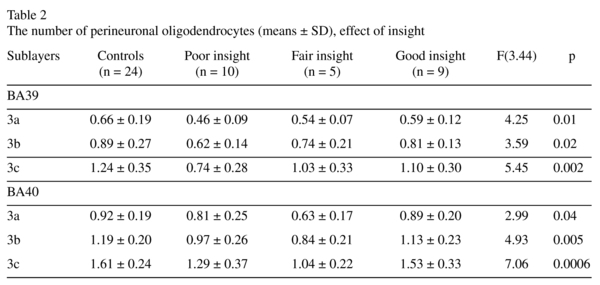
There was a significant effect of insight on the number of PnOl in both BA39 and BA40. In BA 39 the subgroup with poor insight had a significantly lower number of PnOl in each of three sublayers of layer 3 [23-33%, F(3,44) ≥ 3.0, p<0.02] as compared to the control group (Fig. 3) but there were no significant differences between the three insight subgroups. In BA 40 the number of PnOl in the subgroups of subjects with both poor insight and fair insight were significantly lower than in the control group (p<0.02). The subjects with fair insight also had significantly lower number of PnOl than subjects with good insight (p<0.02) (Fig. 3). Comparing the results of this study with the results of our previous study on these same sections, the number of PnOl correlated significantly with the numerical density of oligodendrocyte clusters in three sublayers of layer 3 in BA39 in both control and schizophrenia groups and in BA40 (sublayer c) in the control group but not in the schizophrenia group (Fig. 4).
Discussion
Patients versus controls
We found a significant deficit of PnOl in all sublayers of layer 3 in both BA39 (-23%) and BA40 (-17%) in the schizophrenia group as compared to the control group. The number of PnOl was not correlated with postmortem interval, refrigerator interval, brain weight, brain pH, age at onset or duration of disease in BA 39 and BA 40.
There was no effect of total lifetime antipsychotics on the number of PnOl. However, antipsychotics have been shown to promote the differentiation of oligodendrocyte progenitor cells by regulating oligodendrocyte lineage transcription factors 1 and 217. They have also been shown to improve myelin/oligodendrocyte-related dysfunction18, and to enhance oligodendrocyte regeneration and myelin repair after cuprizone-induced demyelination19. Together these data would indicate that the decrease in the number of PnOl to not be due to the effects of medication but rather to be associated with the diagnosis of schizophrenia.
We applied a two-dimensional method for the assessment of the number of PnOl. Although this method does not allow for the counting of all oligodendrocytes adjacent to neurons, it does allow for successful comparing of this parameter between the control and disease groups, and our current data concurs with data previously obtained by the stereological optical dissector method6 showing the deficit of the numerical density of oligodendrocytes in layer 3 of the IPL (BA39). The present data are also consistent with the reduced number of PnOl found in layer 3 of the dorsolateral PFC in schizophrenia15, and with the reduction of the numerical density of oligodendrocyte clusters found in the IPL in schizophrenia that was associated with insight in schizophrenia7. The findings of this study are also consistent with the results of Byne et al.20 who reported a decrease in the ratio of oligodendrocytes to neurons in the anterior thalamic nucleus in schizophrenia.
Kim and Webster21,22 using a genome-wide correlation analysis explored the genes and biological processes associated with the number of PnOl in the PFC in schizophrenia (using the data from our previous study15). A correlation analysis between genome-wide expression levels and cytoarchitectural traits revealed that 818 genes were significantly correlated with a decrease in the number of PnOl in schizophrenia subjects as well as in the subjects with bipolar disorder and major depression. Several oligodendrocyte-related mRNA, including the mRNA levels for the schizophrenia susceptibility gene ERBB3, showed significant correlations with the number of PnOl. Genome-wide correlation analysis also identified apoptosis as one of the potential mechanisms that may underlie the decrease in the number of PnOl in the PFC of subjects with schizophrenia. The authors suggest that apoptosis, perhaps associated with cytotoxicity mediated by a glutamate abnormality, may contribute to the reduced numbers of PnOl in the PFC of subjects with major psychiatric disorders. A further analysis23 also indicated that several SNPs, including one associated with the glutamine transporter gene SLC38A1 and one with RAB-2A, were associated with the number of PnOl thus providing novel insights into the possible genetic causalities associated with the deficit of PnOl in schizophrenia and affective disorders.
Lack of asymmetry of PnOl in schizophrenia
The present study showed that the number of PnOl was significantly higher in the left hemisphere than in the right hemisphere in all three sublayers of BA39 in the control group but not in the schizophrenia group. This data is consistent with the results of the previous study that demonstrated a significant lateralization of oligodendrocyte density in layer 3 of the IPL (BA39) (L>R) in the control group but not in the schizophrenia group6. The IPL is among the most highly lateralized areas of the brain, and both decreased cerebral lateralization and reversed asymmetry of the IPL, particularly in the angular gyrus, have been reported in schizophrenia10-13. Together with the results of the present study, these data provide evidence for the involvement of the oligodendrocytes and of PnOl in particular in the decreased lateralization of the angular gyrus (BA39) in schizophrenia.
Effects of insight
We found that in BA39 the number of PnOl was significantly decreased in the schizophrenia subgroup with poor insight as compared to the control group. In BA40 the number of PnOl was significantly decreased in the subgroups with both poor and fair insight as compared to controls. However, in both areas BA39 and BA40 schizophrenia subjects with poor insight did not differ from subjects with good insight. Although in BA40 the subgroup with fair insight had a significantly lower number of PnOl than subjects with good insight. Thus, the present study shows for the first time that the decreased number of PnOl in the IPL in schizophrenia is associated with the patient's lack of insight into their disorder. The data are important because the IPL is implicated in neurocognitive impairment in schizophrenia8 and cognitive insight can be a useful prognostic indicator, and thus should be considered in future studies and as a potential focus for treatment24.
Density of PnOl and oligodendrocyte clustering in schizophrenia
In BA39 we found decreased number of PnOl (-23%), we previously found that the numerical density of oligodendrocyte clusters was also decreased (-23%)7. Moreover, in BA39 the number of PnOl correlated significantly with the numerical density of oligodendrocyte clusters in three sublayers of layer 3 in both control and schizophrenia groups. However, in BA40 the correlations were only in the control group but not in the schizophrenia group. Szuchet et al.25 in molecular genetic study showed that cortical perineuronal cells are the progeny of oligodendrocyte progenitors and, hence, are member of the oligodendrocyte lineage. Recent experimental data demonstrated that cell clusters in adult rodent and primate brain contain oligodendrocyte progenitors at different stages of maturation26,27.
In addition cell cycle abnormalities and incomplete differentiation of oligodendrocytes have been reported in schizophrenia28-30. Together these data suggest that the reduced number of PnOl in schizophrenia may be associated with the reduced proliferation of oligodendrocyte progenitors. Recent cytochemical and cytological data suggest that PnOl in the somatosensory cortex support neuronal survival, differentiation, and function, and that they provide protection against neuronal apoptosis (Takasaki et al., 2010)31. Thus, the significant decrease in the number of PnOl may have negative consequence for neuronal activity. In conclusion, the results of the present study suggest that normal oligodendroglia-neuronal relationships in the IPL are likely dysregulated in subjects with schizophrenia primarily due to a decrease of the number of PnOl. The deficit of PnOl is associated with impaired insight and may play a key role in the pathophysiology of schizophrenia. It may also have important implications for the development of treatment strategies.
Authors' contributions
Dr. Vostrikov designed the study, carried out data collection and interpretation and wrote the manuscript. Dr. Kolomeets contributed to the study design, data collection and interpretation, and preparation of the manuscript. Dr. Uranova designed the study, performed statistical analysis, wrote and revised the manuscript.
Acknowledgments
The authors would like to thank the Stanley Medical Research Institute for support this work. Postmortem brain sections were donated by Dr. M. J. Webster from the Stanley 'Parietal Collection'. We express our gratitude to Dr. E. Fuller Torrey for examination of clinical records and editing the manuscript and Dr. M.J. Webster for editing the manuscript.
References
1. Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol 2011; 93: 13-24. [ Links ]
2. Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in neuroscience 2008; 31: 361-370. [ Links ]
3. Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull 2001; 55: 597-610. [ Links ]
4. Uranova NA, Vikhreva OV, Rachmanova VI, Orlovs-kaya DD. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: A postmortem morphometric study. Schizophr Res and Treatment 2011; 2011: 325789. [ Links ]
5. Vostrikov V, Orlovskaya D, Uranova N. Deficit of pericapillary oligodendrocytes in the prefrontal cortex in schizophrenia. World J Biol Psychiatr 2008; 9: 34-42. [ Links ]
6. Vostrikov VM, Kolomeets NS, Uranova NA. Reduced oligodendroglial density in neocortex and lack of insight in schizophrenia. Eur J Psychiat 2013; 27: 111-121. [ Links ]
7. Kolomeets NS, Vostrikov VM, Uranova NA. Abnormalities in oligodendrocyte clusters in the inferior parietal cortex in schizophrenia are associated with insight schizophrenia. Eur J Psychiat 2013; 27: 248-258. [ Links ]
8. van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol MJ, Nolen WA, et al. Insight in schizophrenia: involvement of self-reflection networks? Schizophr Bull 2013; 39: 1288-1295. [ Links ]
9. Tu PC, Lee YC, Chen YS, Li CT, Su TP. Schizophrenia and the brain's control network: aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr Res 2013; 147: 339-347. [ Links ]
10. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res 2007; 97: 215-225. [ Links ]
11. Smiley JF, Konnova K, Bleiwas C. Cortical thickness, neuron density and size in the inferior parietal lobe in schizophrenia. Schizophr Res 2012; 136: 43-50. [ Links ]
12. Niznikiewicz M, Donnino R, McCarley RW, Nestor PG, Iosifescu DV, O'Donnell B, et al. Abnormal angular gyrus asymmetry in schizophrenia. Am J Psychiatr 2000; 157: 428-437. [ Links ]
13. Swanson N, Eichele T, Pearlson G, Kiehl K, Yu Q, Calhoun VD. Lateral differences in the default mode network in healthy controls and patients with schizophrenia. Hum Brain Mapp 2011; 32: 654-664. [ Links ]
14. Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol 1984; 228: 105-116. [ Links ]
15. Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res 2007; 94: 273-280. [ Links ]
16. Zillles K. Architecture of the human cerebral cortex. In: Paxinos G, Mai JK, eds. The human nervous system. 2nd edition. Elsevier Academic Press; p. 997-1055. [ Links ]
17. Fang F, Zhang H, Zhang Y, Xu H, Huang Q, Adilijiang A, et al. Antipsychotics promote the differentiation of oligodendrocyte progenitor cells by regulating oligodendrocyte lineage transcription factors 1 and 2. Life Sci 2013; 2013; 93: 429-434. [ Links ]
18. Ren Y, Wang H, Xiao L. Improving myelin/oligoden-drocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol 2013; 16: 691-700. [ Links ]
19. Zhang Y, Zhang H, Wang L, Jiang W, Xu H, Xiao L, et al. Quetiapine enhances oligodendrocyte regeneration and myelin repair after cuprizone-induced demyelination. Schizophr Res 2012; 138: 8-17. [ Links ]
20. Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchsbaum MS, Haroutunian V. Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res 2006; 85: 245-253. [ Links ]
21. Kim S, Webster MJ. Correlation analysis between genome-wide expression profiles and cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. Mol Psychiatry 2010; 15: 326-336. [ Links ]
22. Kim S, Webster MJ. The stanley neuropathology consortium integrative database: a novel, web-based tool for exploring neuropathological markers in psychiatric disorders and the biological processes associated with abnormalities of those markers. Neuropsychopharmacology 2010; 35: 473-482. [ Links ]
23. Kim S, Webster MJ. Integrative genome-wide association analysis of cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. Mol Psychiatry 2011; 16: 452-461. [ Links ]
24. O'Connor JA, Wiffen B, Diforti M, Ferraro L, Joseph C, Kolliakou A, et al. Neuropsychological, clinical and cognitive insight predictors of outcome in a first episode psychosis study. Schizophr Res 2013; 149: 70-76. [ Links ]
25. Szuchet S, Nielsen JA, Lovas G, Domowicz MS, de Velasco JM, Maric D, et al. The genetic signature of perineuronal oligodendrocytes reveals their unique phenotype. Eur J Neurosci 2011; 34: 1906-1922. [ Links ]
26. Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010; 68: 668-681. [ Links ]
27. Peters A, Sethares C. Oligodendrocytes, their progenitors and other neuroglial cells in the aging primate cerebral cortex. Cerebral Cortex 2004; 14: 995-1007. [ Links ]
28. Radu A, Hristescu G, Katsel P, Haroutunian V, Davis KL. Microarray database mining and cell differentiation defects in schizophrenia. Adv Exp Med Biol 2011; 696: 67-74. [ Links ]
29. Katsel P, Davis KL, Li C, Tan W, Greenstein E, Kleiner Hoffman LB, et al. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology 2008; 33: 2993-3009. [ Links ]
30. Kerns DC, Vong GS, Barley K, Dracheva S, Katsel P, Casaccia P, et al. Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophr Res 2010; 120: 150-158. [ Links ]
31. Takasaki C, Yamasaki M, Uchigashima M, Konno K, Yanagawa Y, Watanabe M. Cytochemical and cytological properties of perineuronal oligodendrocytes in the mouse cortex. Eur J Neurosci 2010; 32: 1326-1336. [ Links ]
![]() Correspondence:
Correspondence:
Victor M. Vostrikov
Laboratory of Clinical Neuropathology
Mental Health Research Center
Zagorodnoe shosse 2
117152, Moscow, Russia
Tel: +7-495-952-87-70
Fax: +7-495-952-89-40
E-mail: vostrikovvm@mail.ru
Received: 24 December 2013
Revised: 20 March 2014
Accepted: 22 March 2014