Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Oncología (Barcelona)
versión impresa ISSN 0378-4835
Oncología (Barc.) vol.29 no.10 dic. 2006
REVISIÓN
The usefulness of reirradiation in the treatment of pelvic recurrence of rectal and gynaecological tumours
F. J. Andreu Martínez; J. M. Martínez Mateu; R. Cardenal Macía
Servicio de Oncología Radioterapica. Hospital Universitari Sant Joan. Alicante (España)
SUMMARY
Pelvic tumors recurrences have diminished with the modern radiation therapy techniques. However, they are still observed, with or without distant metastases, leading to a serious and even mortal disease. The choice of an adequate therapy poses a clinical challenge. One of the procedures is the repetition of radiation therapy.
In this review we manifest how complex is the treatment of gynecological and rectal tumors recidivations, and the importance of a detailed consideration of the available therapies in order to make a decision, specially when the primary tumor has been previously irradiated, be it as only treatment or combined with other therapies.
Key words: Local recurrence. Reirradiation. Retreatment. Radiotherapy. Rectal cancer. Gynecological tumors.
RESUMEN
La recidiva local de tumores pélvicos tras irradiación, aunque ha disminuido su incidencia gracias a las modernas técnicas de radioterapia, sigue produciéndose acompañada o no de metástasis a distancia. Además dicha recidiva ocasiona una gran morbilidad e incluso puede ser la causa de la muerte de estos pacientes. La selección del método de retratamiento a emplear representa un importante desafío clínico. De todos los métodos de retratamiento posibles a utilizar, la reirradiación es uno de ellos.
En esta revisión intentamos poner de manifiesto la complejidad del tratamiento en el caso de tumores ginecológicos y rectales recidivados y lo importante de considerar minuciosamente todas las alternativas terapéuticas que existen, para poder tomar una decisión final adecuada, sobre todo, si los pacientes ya han sido previamente irradiados como tratamiento único o combinado de su tumor primario.
Palabras clave: Recidiva local. Reirradiación. Retratamiento. Radioterapia. Cáncer de recto. Tumores ginecológicos.
Introduction
Although with improvement of radiotherapy (RT) techniques and increase in dose delivered to the pelvic tumour has improved local control, local pelvic recurrence after radiotherapy still occurs (with or without distant metastasis), and such recurrence can be distressing or even fatal1.
Selection of the retreatment method to relieve symptoms or improve the prognosis is important, but this remains a difficult management issue and represents a significant challenge2. Despite this interest and the experience of many radiation oncologists, there have been few reports dealing with reirradiation3.
Pelvic reirradiation is a local treatment with radiotherapy in patients with loco-regional recurrence of pelvic tumours previously treated with radiation therapy. The aim of the treatment is giving high dose of radiation in target volume and diminishing the radiation doses in normal tissues and critics organs.
Reirradiation is one of the possible methods, and others isolates treatments could be surgery or chemotherapy, but nowadays an adequate combination of them may get the best results.
Conventional thinking in radiation oncology has been that a heavily irradiated tissue will not tolerate retreatment, but recently this idea has lost strength. First of all, it is very important to analyze the techniques used in the initial treatment (beam energy, volume. doses delivered with external or intracavitary irradiation). Also, the period of time between the two treatments must be taken in consideration because is postulated that some repair of the initial damage may take place in the interval. However, it is foolhardy to assume that previously irradiated tissues will have the same tolerance as newly irradiated tissues. Factors that determine the extent to which residual injury will limit retreatment tolerance include4, 5: a) the amount of cell depletion caused by prior treatment, b) the time elapsed since that treatment (and therefore the extent of regeneration), c) the tissue at risk. High prior doses, short intervals between treatment courses and slow regeneration of target cells will reduce retreatment tolerance. Reirradiation of previously irradiated patient may be possible, nevertheless, must be undertaken with caution.
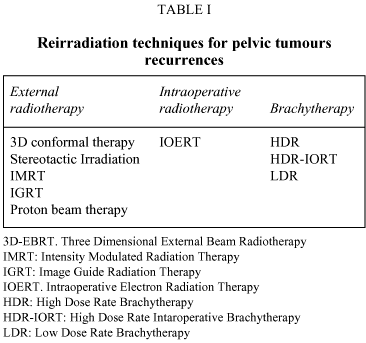
The most important reirradiation techniques for loco-regional pelvic tumours recurrence are shown in Table I and the most frequently reirradiated pelvic tumours are: rectal carcinoma and gynaecological tumours, as cervix and endometrial cancer.
Rectal carcinoma
Local recurrence of rectal cancer after combined radiation and adequate surgery is fortunately rare3. The survival of patients with recurrent or metastatic colorectal cancer usually is less than 12 months1. It is difficult to manage and many of these tumours adhere to or invade into vital pelvic structures rendering surgery or external beam radiotherapy as palliative treatment.
Mohiuddin et al6, 7 from University of Kentucky Medical Center published in 2000 the results in 103 patients with recurrent adenocarcinoma of the rectum underwent reirradiation with concurrent 5-fluorouracil (5FU)-based chemotherapy. The median time from initial treatment to recurrence was 19 months; all the patients were previously irradiated with a median initial radiation dose to the pelvis of 50,4 Gy. Irradiation techniques consisted of two lateral fields with/without a posterior pelvic field to include recurrent tumour with a margin of 2-4 cm only. Total cumulative doses ranged from 70 to 100 Gy with a median total dose of 85 Gy. After the reirradiation, 34 patients also underwent surgical resection for residual disease. With a median follow-up of 2 years, the median survival for the whole group was 26 months and the 5-year actuarial survival rate was 19%. Nevertheless, the group of patients undergoing surgical resection after reirradiation had a median interval and 5-year survival rate of 44 months and 22% compared with 14 months and 15% for patients treated with reirradiation only (p= 0.0001). Late complications were seen in 22 patients, including persistent severe diarrhoea in 18 patients, small bowel obstruction was seen in 15 patients, fistula formation in 4 patients, and coloanal stricture in 2 patients.
Haddock et al8 from Mayo Clinic and Mayo Medical School published in 2001 the results in 51 previously irradiated patients with locally advanced colorectal cancer without evidence of distant metastatic disease who were treated with surgical resection before intraoperative electron radiotherapy (IOERT) +/- additional external beam radiotherapy (EBRT). Surgery was made to achieve a gross total resection (R1 or R0) before IOERT if it could be safely accomplished. The median IOERT dose was 20 Gy (range from 10 to 30 Gy). 37 patients received additional EBRT either pre or postoperatively (median 25,2 Gy). 21 patients received chemotherapy with 5-FU ± leucovorin during radiotherapy but only 13 received additional cycles of 5-FU ± leucovorin as maintenance chemotherapy. The median, two year and five year actuarial overall survivals were 23 months, 48% and 12%, respectively. The 2-year actuarial central control (within IOERT field) is 72%. There is a trend toward improved local control in patients who received as least 30 Gy EBRT in addition to IOERT as compared to those who received no EBRT or less than 30 Gy with 2-year local control rate of 81% versus 54%. The main IORT related toxicity was peripheral neuropathy, sixteen (32%) patients developed neuropathies. Ureteral narrowing or obstruction occurred in seven patients too. But long term survival is poor due to the high rate of distant metastasis.
High dose rate intraoperative brachytherapy (HDR-IORT) is used for two authors in order to treat recurrent colorectal cancer.
One of them is Nag et al1 from Division of Radiation Oncology; Arthur G James Cancer Hospital and Research Institute. They in an attempt to improve the fatal prognosis of recurrent or metastatic colorectal carcinoma investigated the role of HDR-IORT in the management of these patients. 26 patients were treated with maximal surgical resection and HDR-IORT. They included several patients who were previously irradiated. Intraoperative radiation dose ranged from 10 to 20 Gy. The surgical resection was microscopic (R1) in 16 patients (62%) and gross residual (R2) in 10 patients (38%). 6 patients received postoperative EBRT too. After a median follow-up of 28 months, seven of 15 evaluable patients (47%) failed in the area treated with HDR-IORT. The median survival was 23 months (R1 24 months; R2 17 months), with a 4-year actuarial survival rate of 36%. They concluded that the use of HDR-IORT in association with radical resection increases local control in patients with recurrent or metastatic colorectal cancer and patients with microscopic residual disease achieved a better result than do those with gross residual disease.
The other is Alektiar et al9 from Memorial Sloan-Kettering Cancer Center, this article is an update of their preliminary report with longer follow-up and larger patients number. Seventy four patients with locally recurrent rectal cancer were treated with surgery plus HDR-IORT. Additional EBRT was given to 29 patients, and 33 patients received 5 fluorouracil based chemotherapy. All the patients underwent complete gross resection, and 21 of 74 had positive microscopic margin. The dose of HDR-IORT ranges from 10 to 18 Gy. With a median follow-up of 22 months, the 5-year local control, and overall survival rates were 39% and 23% respectively. They conclude that for overall survival a negative microscopic margin (p= 0.04) and the use of HDR-IORT + EBRT (p= 0.04) were significant predictors of improved survival, nevertheless, the only predictor of improved local control was a negative margin of resection with a 5-year local control rate of 43%, compared to 26% in those with positive margin (p = 0.02) The incidence of peripheral neuropathy was 16%.
Glimelius3 from University Hospital Uppsala reported that if possible to reirradiated patients who developed recurrence and have already had pre or postoperative radiotherapy to a dose of about 50 Gy over five weeks or 25 Gy in one week. They could be treated up to al least 30 Gy externally over three weeks. In addition, IOERT or HDR-IORT may be given. This strategy may give benefit but cure is not possible unless the recurrence is surgically resectable.
Finally Watson et al10 from University of Michigan Medical Center arrived at the conclusion in 1996 that reirradition of previously irradiated rectal cancer would be considered only when there is no other alternative for effective therapy and in the face of progressive and severe symptoms. They recommended the use of three dimensional treatment planning, portals can be designed to limit dose to previously irradiated critical structures while minimizing the risk of treatment related complications.
Results of the different treatments are summarized in Table II.
Gynaecological tumours
Local control rates in advanced cervical cancer has improved dramatically over the last two decades with the widespread used of standardized brachytherapy and, more recently, with the addition of concurrent cisplatin. However some 20 to 40% of patients with advanced primary tumours are still expected to recur locally11. Local control prospects are even worse for patients who present with recurrent disease, especially if they have received radiotherapy during the primary management. Whereas 25 to 50% of selected patients with gynaecologic tumours who relapse centrally in an irradiated pelvis can be salvaged by exenteration, postirradiation recurrences infiltrating the pelvic side wall are not usually considered candidates for exenterative surgery because tumour-free margins cannot be achieved and generally the prognosis of these patients have been fatal, although based on the biologic factors of the tumour, 50 per cent might be salvaged if local control is achieved12.
Mahé et al13 in 1996 reported the results of the French intraoperative radiation therapy group; they retrospectively reviewed data from a cohort of 70 nonselected patients with pelvic recurrence of cervical carcinoma and treated in seven French institutions with IOERT. Fifty four patients have received previously radiotherapy (11 radiotherapy alone and 43 radiotherapy plus surgery). The initial treatment with radiotherapy was usually a combination of EBRT plus brachytherapy. Nevertheless only four patients were given neoadjuvant chemotherapy (CT) with 5FU and cisplatin. The mean time to recurrence was 70 months (range 3-288 months) and the location of pelvic failure was the sidewall (± central pelvis) in 59 out of 70 patients (84%) and the central pelvis alone in 11 out of 70 (16%). The modalities of treatments for recurrences were surgery plus IOERT in 40 patients, surgery plus IOERT plus EBRT in 30 patients. Twenty patients received additional CT before or after IOERT, the protocol was usually a combination of 5FU and cisplatin or a cisplatin-containing regimen. The kinds of resection were gross complete in 30 patients, gross incomplete or biopsy in 37 patients and not related in 3 patients. The mean IOERT dose was 18 Gy (range from 10 to 25 Gy) when gross complete resection was done and 19 Gy (range from 10 to 30 Gy) when partial resection (gross incomplete resection or biopsy) was done. Mean follow-up after IOERT was 15 months (range 2-69 months). Median survival was 11 months and local control 21%. One, 2- and 3-year overall survival rates were 47, 17 and 8%, respectively. Although median survival, local control increased after gross complete resection (13 months, 27%) versus partial resection (10 months, 16%) and median survival, local control and overall survival at one, 2- and 3-year increased when initial treatment consisted of surgery (S) alone (15 months; 25%; 56%, 31% and 19%) versus radiotherapy (RT±S) (10 months; 16%; 30%, 11% and 4%), results are shown too in Figure 1. However, these differences were not statistically significant. No death-related toxicity was observed. Grade 2 or 3 toxicity was observed in 19 out 70 patients (27%), including 9 not directly IOERT-related complications (13%; three digestive tract fistulas, one rectal stricture, three urinary fistulas, two infections) and 10 directly IOERT-related complications (14%; five neuropathies, four ureteral obstructions and one rectal stricture).

Martínez-Monge et al14 from Clinica Universitaria of Navarra in 2001 reported their results for locally advanced and recurrent cervical cancer. 67 patients were treated with IOERT. Thirty-six had recurrent cervical cancer and thirty-one had primary locally advanced disease. 31 out of 36 patients with recurrent disease occurring in a previously irradiated area were evaluated for radical surgery as initial option, unless their disease was considered unresectable (determined by the presence of tumour fixation to the pelvic boundaries by gynaecological examination and, in some borderline cases, with the aid of pelvic MRI). They were revaluated for surgery after they received two or three courses of chemotherapy usually a combination of cisplatin, adriamycin and ifosfamide. Surgery resections were with gross residual disease in 5 patients, microscopic residual disease in 10 patients and no residual disease in 21 patients. The median dose of EBRT that these patients received previously was 53 Gy (range from 40 to 75 Gy) and IOERT median dose was 15 Gy for recurrent disease (range from 10 to 20 Gy). After median follow-ups of 18,9 months for patients with recurrent disease, the 10 year disease free survival and overall survival for these patients were 16% and 14% respectively, nevertheless the 10 year overall survival for patients with gross residual disease was 0% (median survival time: 7,8 months), for patients with microscopic disease was 9% (median survival time: 11,1 months), and for patients with no residual disease was 45,3% (median survival time: 61,1 months). The overall incidence of toxic events which are not usually observed after radical surgery and that might attributable to IOERT was 14,9%. Chronic pain was observed in 6 patients (8,9%) in the recurrent group.
Hockel et al12 from University of Mainz reported their experience with 48 patients with postirradiation recurrent or persistent gynaecologic malignancies. They have designed the combined operative and radiotherapeutic treatment (CORT) procedure for the treatment of postirradiation recurrence infiltrating the pelvic wall and developed several new techniques for its realization. After the surgery procedure (R1), the tumour bed is irradiated with brachytherapy of HDR. At a median follow-up of 33 months (range from 3 to 71 months), the 5-year survival probability was 44%. The overall control rate was 68% and 85% in the last 25 patients in the series. The severe complications rate at 5 years was 33%. They concluded that R1 resection of the tumour at the pelvic wall is mandatory in order to get long term survival.
In this way Abe and Shibamoto15 in 1996 concluded that the results of IORT for previously irradiated patients are most discouraging, and the gross surgical resection is mandatory in patients with cervical cancer recurrence previously irradiated if we want to achieve a survival benefit. Peripheral neuropathy and ureteral stenosis constitute the major toxicity of pelvic IORT.
Finally Zuliani de Oliveira et al in 200516 reported their results with 11 patients diagnosed of cervical carcinoma who presented pelvic recurrence after radiation therapy and were treated with HDR. The median follow-up was 22.5 months (2 to 54 months). Ten patients (91%) presented complete clinical response, three patients (27%) were disease free, two were alive with disease, three died of cancer and three were lost in the follow-up after the second recurrence. One patient died without evaluation of the response. The major toxicity was urinary tract toxicity grade III in one patient (9%).
Results of the different treatments are summarized in Table III.
Conclusions
The pelvic reirradiation must not be the exclusive standard treatment in patients with recurrent pelvic tumours after a previously course of irradiation. As minimal data is available on the toxicity of additional radiation therapy, this approach would be considered only when there in no other alternative for effective therapy and in the face of progressive and severe symptoms.
The irradiation techniques more frequently used are intraoperative radiotherapy or high dose brachytherapy. Although some institutions have a good experience without prohibitive long-term side effects using three dimensional conformal radiotherapy.
The best results are achieved with the combination of several treatments (surgery, chemotherapy, hyperthermia, etc), especially if the patient was treated initially with radiotherapy.
We hope that the new diagnostic methods, as dynamic MRI or PET that allow to get anatomical, as well biological and functional information of the tumours, in addition of the planning systems and the new treatment techniques (IOERT, IMRT, IGRT, HDR-IORT) will allow to delimitate more exactly the target volume, reducing the dose to the normal tissues and critical structures of the pelvis, in order to get a better local control and minimizing the risk of treatment related complications.
References
1. Nag S, Martinez-Monge R, Mills J, et al. Intraoperative high dose rate brachytherapy in recurrent or metastatic colorectal carcinoma. Ann Surg Oncol. 1998; 5: 16-22. [ Links ]
2. Alektiar KM, Zelefsky MJ, Paty PB, et al. High-dose-rate intraoperative brachytherapy for recurrent colorectal cancer. Int J Radiat Oncol Biol Phy. 2000; 48: 219-26. [ Links ]
3. Glimelius B. Recurrent rectal cancer. The pre-irradiated primary tumours: can more radiotherapy be given?. Colorectal Dis. 2003; 5: 501-3. [ Links ]
4. Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin Radiat Oncol. 2000; 10: 300. [ Links ]
5. Withers HR, Mc Bride WH. Radiation effects on normal tissues. Front Radiat Oncol. 1999; 22: 1. [ Links ]
6. Lingareddy V, Ahmad NR, Mohiuddin M. Palliative reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phy. 1997; 38: 785-90. [ Links ]
7. Mohiuddin M, Marks G, Marks J. Long-term results or reirradiation for patients with recurrent rectal carcinoma. Cancer. 2000; 95: 1144-50. [ Links ]
8. Haddock MG, Gunderson LL, Nelson H, et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phy. 2001; 49: 1267-74. [ Links ]
9. Alektiar KM, Zelefsky MJ, Paty PB, et al. High dose rate intraoperative brachytherapy for recurrent colorectal cancer. Int J Radiat Oncol Biol Phy. 2000; 48: 219-26. [ Links ]
10. Watson B, Robertson JM, Marsh L, et al. A three-dimensional approach for re-irradiation of recurrent colorectal adenocarcinoma. Med Dosi. 1996; 21: 79-82. [ Links ]
11. Andreu-Martínez FJ, Martínez-Mateu JM. Hypoxia and anaemia in patients with cancer of the uterine cervix. Clin Transl Oncol. 2005; 7: 323-31. [ Links ]
12. Hockel M, Sclenger K, Hamm H, et al. Five-year experience with combined operative and radiotherapeutic treatment of recurrent gynecologic tumours infiltrating the pelvic wall. Cancer. 1996; 77: 1918-33. [ Links ]
13. Mahé MC, Gérard JP, Dubois JB, et al. Intraoperative radiation therapy in recurrent carcinoma of the uterine cervix: reports of the French intraoperative group on 70 patients. Int J Radiat Oncol Biol Phy. 1996; 34: 21-6. [ Links ]
14. Martínez-Monge R, Jurado M, Aristu JJ, et al. Intraoperative Electron Beam Radiotherapy during Radical Surgery for Locally Advanced and Recurrent Cervical Cancer. Gynecol Oncol. 2001; 82: 538-43. [ Links ]
15. Abe M and Shibamoto Y. The usefulness of intraoperative radiation therapy in the treatment of pelvic recurrence of cervical cancer. Int J Radiat Oncol Biol Phy.1996; 34: 513-4. [ Links ]
16. Zuliani de Oliveira AC, Barros Esteves SC, Andrade Feijo LF et al. Braquiterapia iIntersticial para recidivas de câncer de colo uterino pós-radioterapia. Radiol Bras. 2005; 38: 117-20 [ Links ]
 Correspondence:
Correspondence:
Dr. F. J. Andreu Martínez
Servicio de Oncología Radioterápica
Hospital Universitari Sant Joan
Crta. Ncal. 332 Alicante-Valencia, s/n.
E-03550 Sant Joan d'Alacant (Alicante)
España
andreu_fra@gva.es.
Recibido: 04.04.06
Revisado: 10.04.06
Aceptado: 29.05.06














