Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.5 Madrid may. 2004
| ORIGINAL PAPERS |
Safety and efficacy of argon plasma coagulator ablation therapy for flat
colorectal adenomas
A. García, O. Núñez, C. González-Asanza, A. Parera, L. Menchén, C. Ripoll, C. Senent, E. Cos and P. Menchén
Unit of Gastrointestinal Endoscopy. Department of Digestives Diseases. Hospital Gregorio Marañón. Madrid, Spain
ABSTRACT
Introduction: argon-plasma coagulation (APC) has been used safely and efficaciously in multiple settings including colon polyp treatment. The aim of this study was to evaluate APC efficacy and safety in the treatment of flat colorectal adenomas.
Materials and methods: APC ablation was prospectively performed and evaluated in 22 consecutive patients with colorectal adenomas, 11 of which had large sessile adenomas that were treated with piecemeal polypectomy and APC ablation of residual adenomatous tissue, whereas the remaining eleven patients with flat or carpet-like adenomas were only treated with APC. The mean initial longitudinal extension of adenomas to be treated with APC was 22 mm (range, 20 to 40 mm).
Results: the mean age of patients was 70 years. Adenomas were found most frequently in the rectum (50%) and cecum (23%). Complete ablation was achieved in 90.9% of adenomas. Recurrence was observed in 20% of patients, all of them in the rectum, after a mean follow-up period of 16.3 months (range, 8 to 35). All recurrences were managed satisfactorily. No major complications were seen.
Conclusions: argon plasma coagulator ablation of flat colorectal adenomas is an efficacious and safe technique, specially in the right colon, but results must be confirmed in controlled trials with a higher number of patients.
Key words: Argon-plasma coagulation. Colorectal adenomas.
García A, Núñez O, González-Asanza C, Parera A, Menchén L, Ripoll C, Senent C, Cos E, Menchén P. Safety and efficacy of argon plasma coagulator ablation therapy for flat colorectal adenomas. Rev Esp Enferm Dig 2004; 96: 315-321.
Recibido: 10-04-03.
Aceptado: 15-12-03.
Correspondencia: Óscar Núñez Martínez. Servicio de Aparato Digestivo. Hospital General Universitario Gregorio Marañón. C/ Dr. Esquerdo, 46. 28007 Madrid. Telf.: 91 586 83 07. Fax: 91 426 50 24. e-mail: onumar@inicia.es
INTRODUCTION
Argon-plasma coagulation (APC) has been used in digestive endoscopy since 1991, when a specific electrode that could be introduced through the endoscope channel was designed (1). It is a monopolar electrosurgical device that does not need physical contact with tissue, as a high-frequency electrical current is transmitted to the tissue by ionized argon gas. APC produces superficial thermal damage about 2-3 mm deep depending on energy output and the time current is applied (2).
Endoscopic APC has been applied in different settings such as: localized or diffuse vascular lesions like angiodysplasia or watermelon stomach (3,4), Barrett's esophagus (5,8), radiation colitis (9-11), bleeding peptic ulcer (4,12), Zenker's diverticulum (13), malignant tumors and specially in repermeabilization of intraluminal growths after pros-thesis placement for the palliative treatment of esophagus cancer (14-16) and colonic polyps (3,4).
Large sessile polyps are more likely to have neoplastic foci, with a high rate of recurrence after excision in previous series (16-46%), as well as a higher frequency of postpolypectomy complications such as bleeding and perforation (17-19). For these reasons, alternative ways of destroying residual tissue with a lower percentage of complications have been researched, usually associated with piecemeal polypectomy. Diverse results have been reported on the use of argon plasma coagulation in this setting (2,20-22).
The aim of this study was to analyze the efficacy of APC ablation therapy for flat and sessile polyps in association or not with previous piecemeal polypectomy in our Endoscopy Unit, and to evaluate the safety of this therapy in clinical practice.
MATERIAL AND METHODS
Thirty-five patients with flat or sessile polyps treated with APC from May 1997 to December 2000 were included in this study. In this group, 13 patients were excluded from the analysis because of one of the following reasons: high-grade dysplasia, follow-up shorter than 6 months, and loss to follow-up.
All procedures were performed on an outpatient basis initially. An oral polyethylene glycol electrolyte lavage solution and occasionally cleansing enemas were used for colonic preparation. An informed consent was obtained from each patient before each procedure. Conscious sedation was performed by an anesthetist using intravenous propofol when unsedated colonoscopy was not tolerated. Pulse oximetry was used for monitorization.
A specific device for argon gas application with a 2.5 L/min gas flow and variable electric power (from 40 to 80 W) was used depending on polyp location (ERBE APC300; ERBE Electromedizin, Tübingen, Germany). APC treatment was administered using a standard single-channel flexible colonoscope (Fujinon 200, Omiya, Japan). The endoscopic polypectomy was performed using a standard snare and predefined electrocautery energy (Endocut; ERBE ICC 200; ERBE Electromedizin, Tübingen, Germany). Adenomatous tissue extension was measured and compared using open biopsy forceps.
APC treatment was scheduled every 15 days until adenomatous tissue had completely disappeared. Afterwards, a colonoscopic review was done first at 6 months and then annually. Colonoscopic examinations were recorded so that polyp localization was rendered easier on follow-up. Biopsies ware taken in the treated area with or without macroscopic adenomatous tissue in each endoscopic revision.
The endoscopic treatment applied was APC in 11 patients with flat or carpet-like adenomas, while the remaining 11 patients with large sessile adenomas were first treated with piecemeal polypectomy using an electrocautery snare and then completed with APC on residual tissue (Fig. 1). We based our definition of flat adenoma on previously proposed criteria (23,24). The mean initial longitudinal extension of the area treated with APC was 22 mm (20-40). Previous cold forceps biopsies were taken in those patients who were treated only with APC, while the tissue of those polyps partially resected was sent for histological examination.
Complete response was defined as the absence of visible adenomatous tissue both macroscopically and in the histological examination of biopsies taken during endoscopy at follow-up. Recurrence was defined as the presence of new proliferation of adenomatous tissue in areas of previously treated mucosa with documented complete response. Treatment failure was defined as a persistence of a polyp at least half its initial size following three APC sessions.
Qualitative variables expressed as percentages were compared using Fisher's test. Quantitative variables, expressed as means with their range, were compared using Student's t-test. Differences were considered significant when p < 0.05. All statistical analyses were performed using the SPSS software, version 10.0.
RESULTS
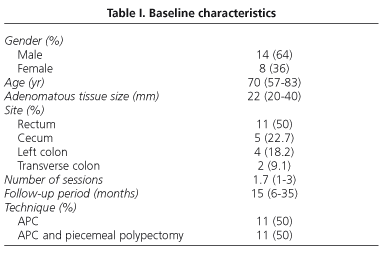
Twenty-two patients were included with a mean follow-up period of 15 months (range, 6 to 35). Adenomas localized in the rectum were most frequent (50%). The characteristics of patients, colorectal polyps and treatments are indicated in table I.
Histological examination revealed a villous component in 86%, and mild or moderate dysplasia in 81% of polyps.
Complete response was obtained in 90.9% of adenomas treated with a mean of 1.7 sessions (range, 1 to 3). Two patients who did not respond to APC after 3 sessions were considered treatment failures. One of the polyps in the rectum was 20-mm long and had been treated with only APC, while the other polyp was 40-mm long and had been treated with piecemeal polypectomy initially, with 20 mm remaining afterwards. Both cases were villous adenomatous polyps with mild dysplasia on histological examination.
Recurrence was detected in 4 patients (20%) after a mean period of 16.3 months (range, 8 to 35 months). All recurrences were observed in rectal polyps. Three cases were managed endoscopically with satisfactory results, but one patient required endoanal surgical resection.
There was no relationship between recurrence and previous piecemeal polypectomy. Only 1 patient out of 10 (10%) who had been treated exclusively with APC recurred, and so did 3 patients (30%) who had been treated with both methods (p > 0.05). Recurrence was related with the initial size of the adenomatous tissue to be treated. Thus, those that recurred had an initial mean extension of 23.8 ± 7.5 mm, while those that maintained a complete remission had a size of 13.2 ± 7 mm (p = 0.01).
No major complications related with the procedure occurred. One patient, after the first session of APC for a cecal polyp, developed inflammatory polyps in the treated mucosa, which disappeared in the following revisions. Two additional patients suffered from transient abdominal pain, which was managed on an outpatient basis.
DISCUSSION
Argon-plasma coagulation is based on the transmission of a high-frequency current through argon gas to result in thermal damage. It has been safely and efficaciously used in multiple settings (3-16).
The rational basis for the use of APC in the treatment of colorectal polyps is ablation of residual adenomatous tissue after piecemeal polypectomy of big polyps and stalk hemostasia. Zlatanic et al. (20) studied the recurrence of adenomatous tissue after piecemeal polypectomy, and observed that recurrence occurred in all cases where residual tissue remained, and in 46 and 50% of cases, respectively, with no residual adenomatous remnants or where APC was applied. In a most recent randomized, controlled study of recurrence prevention after piecemeal resection, APC administration following complete snare excision was seen to reduce polyp recurrence in spite of the small size of the sample (21). When snare excision was not complete and APC was applied, polyp recurrence was observed in 6 out of 13 patients. In another study that was published in ABSTRACT form (22), adenomatous tissue recurrence following APC was observed in 3.5% of patients after a mean follow-up period of 6 months. All studies concluded that residual adenomatous tissue treatment with APC after piecemeal polypectomy is safe and efficacious.
In our study, APC was used as the first and only treatment of flat polyps, as well as for complete ablation of residual adenomatous tissue after piecemeal polypectomy. Recurrence was observed in 20% of cases after a mean follow-up of 16 months. Although our recurrence rate matches those previously described (3.5-46%), our follow-up period was longer. All recurrences were satisfactorily managed endoscopically except in one case, which required endoanal surgery resection.
Although APC was administered as the initial and only treatment of flat adenomatous polyps, this approach did not determine a higher recurrence rate, which was only related with the initial size of the treated lesion. This finding may be relevant particularly in the right colon, where the colonic wall is thinner and therefore more susceptible to perforation. In our study, 22.7% of polyps treated were located in the cecum, but it was not related with a higher rate of complications.
Other techniques such as laser photocoagulation (25) and endoscopic mucosal resection (26,27) had been already used in this setting with good results, but APC ablation is an easier procedure that is associated with a lower risk of complications, and thus may become a first-choice technique with this indication. However, this hypothesis has still to be evaluated in controlled, randomized clinical trials comparing these modalities of endoscopic treatment for adenoma.
Inflammatory polyps emerged after APC application in one cecal polyp, but they disappeared spontaneously as reported in following controls. This complication had been previously described after APC in the upper gastrointestinal tract (28).
We conclude that the use of APC for the treatment of flat colorectal polyps is safe and efficacious. This finding is specially interesting when the treatment of right-colon and cecum polyps is taken into account, but such results must obtain confirmation in controlled trials with a higher number of patients.
REFERENCES
1. Farin G, Grund KE. Technology of argon plasma coagulation with particular regard to endoscopic applications. Endoscop Surg 1994; 2: 71-7. [ Links ]
2. Watson JP, Bennett MK, Griffin SM, et al. The tissue effect of argon plasma coagulation on esophageal and gastric mucosa. Gastrointest Endosc 2000; 52: 342-5. [ Links ]
3. Johanns W, Luis W, Janssen J, et al. Argon plasma coagulation (APC) in gastroenterology: experimental and clinical experiences. Eur J Gastroenterol Hepatol 1997; 9 (6): 581-7. [ Links ]
4. Wahab PJ, Mulder CJ, den Hartog G, et al. Argon plasma coagulation in flexible gastrointestinal endoscopy: pilot experiences. Endoscopy 1997; 29 (3): 176-81. [ Links ]
5. Kochman ML. Eradication of Barrett's mucosa with argon plasma coagulation and acid suppression: immediate and mid-term results. Gastrointest Endosc 1999; 50 (6): 884-6. [ Links ]
6. May A, Gossner L, Gunter E, et al. Local treatment of early cancer in short Barrett's esophagus by means of argon plasma coagulation: initial experience. Endoscopy 1999; 31 (6): 497-500. [ Links ]
7. Grade AJ, Shah IA, Medlin SM, et al. The efficacy and safety of argon plasma coagulation therapy in Barrett's esophagus. Gastrointest Endosc 1999; 50 (1): 18-22. [ Links ]
8. Van den Boogert J, Van Hillegersberg R, Siersema PD, et al. Endoscopic ablation therapy for Barrett's esophagus with high-grade dysplasia: a review. Am J Gastroenterol 1999; 94 (5): 1153-60. [ Links ]
9. Kaassis M, Oberti E, Burtin P, et al. Argon plasma coagulation for the treatment of hemorrhagic radiation proctitis. Endoscopy 2000; 32 (9): 673-6. [ Links ]
10. Morrow JB, Dumot JA, Vargo JJ. Radiation-induced hemorrhagic colitis treated with argon plasma coagulator. Gastrointest Endosc 2000; 51 (4 Pt 1): 498-9. [ Links ]
11. Silva RA, Correia AJ, Dias LM, et al. Argon plasma coagulation ther-apy for hemorrhagic radiation proctosigmoiditis. Gastrointest Endosc 1999; 50 (2): 221-4. [ Links ]
12. Cipolleta L, Bianco MA, Rotondano G, et al. Prospective comparison of argon plasma coagulator and heater probe in the endoscopic treatment of major peptic ulcer bleeding. Gastrointest Endosc 1998; 48 (2): 191-5. [ Links ]
13. Mulder CJ. Zapping Zenker's diverticulum: gastroscopic treatment. Can J Gastroenterol 1999; 13 (5): 405-7. [ Links ]
14. Akhtar K, Byrne JP, Bancewicz J, et al. Argon beam plasma coagulation in the management of cancers of the esophagus and stomach. Surg Endosc 2000; 14 (12): 1127-30. [ Links ]
15. Watson JP, Bennett MK, Griffin SM, et al. The tissue effect of argon plasma coagulation on esophageal and gastric mucosa. Gastrointest Endosc 2000; 52 (3): 342-5. [ Links ]
16. Grund KE, Storek D, Becker HD. Highly flexible self-expanding meshed metal stents for palliation of malignant esophagogastric obstruction. Endoscopy 1995; 27 (7): 486-94. [ Links ]
17. Binmoeller KF, Bognacker S, Seifert H, et al. Endoscopic snare excision of "giant" colorectal polyps. Gastrointest Endosc 1996; 43: 183-8. [ Links ]
18. Waye JD. How big is to big? Gastrointest Endosc 1996; 43: 256-7. [ Links ]
19. Walsh RM, Ackroyd FW, Shellito PC. Endoscopic resection of large sessile colorectal polyps. Gastrointest Endosc 1992; 38: 303-8. [ Links ]
20. Zlatanic J, Waye JD, Kim PS, et al. Large sessile colonic adenomas: use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc 1999; 49 (6): 731-5. [ Links ]
21. Brooker JC, Saunders BP, Shah SG, et al. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointest Endosc 2002; 55: 371-5. [ Links ]
22. Regula J, Wronska E, Polkwski M, et al. Argon plasma coagulation (APC)after piecemeal polypectomy for colorectal adenomas. Endoscopy 1996; 28: s61-2 (A1274). [ Links ]
23. Sawada T, Hojo K, Moriya Y. Colonoscopic management of focal and early colorectal carcinoma. Balliere's Clinical Gastroenterology 1989; 3: 627-45. [ Links ]
24. Rembacken BJ, Fujii T, Dixon MF, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet 2000; 355: 1211-4. [ Links ]
25. Conio M, Caroli-Bosc FX, Filiberti R, et al. Endoscopic Nd: YAG laser therapy for villous adenomas of the right colon. Gastrointest Endosc 1999; 49: 504-8. [ Links ]
26. Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 2001; 54: 62-6. [ Links ]
27. Ahmad NA, Kochman ML, Long WB, et al. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc 2002: 55: 390-6. [ Links ]
28. Schmeck-Lindenau HJ, Kurtz W, Heine M. Inflammatory polyps: an unreported side effect of argon plasma coagulation. Endoscopy 1998; 30 (8): s93-4. [ Links ]











 texto en
texto en