Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.6 Madrid jun. 2004
| ORIGINAL PAPERS |
Infliximab improves quality of life in the short-term in patients with fistulizing Crohn's disease in clinical practice
V. Cadahia, A. García-Carbonero, S. Vivas1, D. Fuentes, P. Niño, P. Rebollo and L. Rodrigo
Service of Digestive Diseases. Hospital Central de Asturias. Oviedo. 1Section of Digestive Diseases. Hospital Central de León. Spain
ABSTRACT
Objectives: to assess the effect of infliximab on quality of life in a series of patients with fistulizing Crohn's disease.
Patients and methods: a prospective observational study was made. A total of 25 patients with single or multiple draining abdominal or perianal fistulas were selected for the study. All received an intravenous infusion of infliximab at a dose of 5 mg per kilogram of body weight in weeks 0, 2, and 6. The clinical activity was calculated every two weeks over a 10-week follow-up. HRQOL (SF-36 and IBDQ scores ) were compared at baseline and at weeks 4 and 10.
Results: sixty-four percent of patients had a clinical response to treatment with infliximab, with complete closure of fistulas. The mean values of CDAI decreased during follow-up, with a significant difference between weeks 0 and 10 (p < 0.01). Health-related quality of life (HRQOL), as measured by means of SF-36, showed an overall improvement in the physical domain (PCS) after 4 and 10 weeks (p < 0.05). An increase was also observed in IBDQ overall score on comparing the results obtained at week 0 and week 4 (p < 0.01). The social functioning domain of IBDQ was not significantly changed with treatment.
Conclusions: treatment with infliximab in active fistulizing Crohn's disease results in a significant increase in the quality of life of patients at short-term.
Key words: infliximab. Crohn's disease. Health-related quality of life (HRQOL).
Cadahia V, García-Carbonero A, Vivas S, Fuentes D, Niño P, Rebollo P, Rodrigo L. Infliximab improves the quality of life in the short-term in patients with fistulizing Crohn's disease in clinical practice. Rev Esp Enferm Dig 2004; 96: 369-378.
Recibido: 27-10-03.
Aceptado: 15-12-03.
Correspondence: Luis Rodrigo. Servicio de Aparato Digestivo. Hospital Central de Asturias. C/ Celestino Villamil, s/n. 33006 Oviedo. Telf.: 985 10 80 58. Fax: 985 27 36 14.
E-mail: lrodrigos@terra.es
INTRODUCTION
Crohn's disease (CD) is a chronic inflammatory disease of the digestive tube of unknown etiology characterised by segmentary transmural inflammation and occasional granulomatose changes. Although it mainly affects the distal small intestine and the colon, it can affect any part of the gastrointestinal tract from the mouth to the anus. This transmural inflammation penetrates adjoining organs and the skin, with a formation of fistulas. The development of fistulas in CD is a frequent complication, and they arise during the clinical course of the disease in around one third of cases (1,2).
Several therapies have been used for the treatment of fistulous disease based on the use of antibiotics and immunomodulators. However, these have not demonstrated clear effects (3-5). The efficacy of infliximab, a chimeric murine-human monoclonal IgG1 antibody against TNF-alpha has been clearly shown its efficacy in the treatment of CD (6), and infliximab has also proven very efficient in the closure of fistulas (7).
On the other hand, in chronic diseases such as this, where no cure exists, health-related quality of life (HRQOL) is often turned into another therapeutic aim (9). CD is characterised by its chronicity and absence of curative treatment, which together with its different complications causes a considerable impairment of quality of life in these patients (10). Thus, it is currently considered that the treatment of patients with CD must be directed not only toward the management of digestive and systemic symptoms, but also toward improved health-related quality of life.
In this sense, it is important that the extent of HRQOL reduction, and in which dimensions, be known (8). Thus, we can evaluate the various treatments for this disease by studying the changes in HRQOL as expressed by patients treated.
In order to evaluate HRQOL in patients with CD a specific instrument has been designed for this illness, namely "the inflammatory bowel disease questionnaire" (IBDQ). IBDQ was designed by Guyatt et al (11), and its usefulness for assessing HRQOL in patients with CD and ulcerative colitis (UC) has been demonstrated. IBDQ meets the criteria of simplicity and reliability, and reflects disease activity to a better degree. Quality of life has been evaluated by means of IBDQ in controlled clinical trials of infliximab treatment for CD (12-14). However, little is known of the influence of infliximab on the quality of life of these patients in everyday clinical practice.
Data referring to the influence of treatment with infliximab on HRQOL in patients with fistulas, the most incapacitating form of this disease, and which most affects quality of life, are scarce.
The aim of this study was to evaluate the efficacy of infliximab in the treatment of the fistulous form of Crohn's disease, and to demonstrate its influence on the quality of life of these patients in the clinical context and not in controlled clinical trials.
PATIENTS AND METHODS
Patients
An observational prospective study was undertaken in a total of 25 patients with single or multiple draining abdominal or perianal fistulas. These patients were consecutively included between April 2001 and December 2002. All patients were recruited at the Gastroenterology Service of Hospital Central of Asturias. A written informed consent was obtained prior to inclusion in the study.
Patients younger than 18 years of age, those with active infection at the time of inclusion, those who did not receive the complete treatment regimen, and those who did not complete HRQOL questionnaires were excluded.
Methods
All patients had received an unsuccessful immunosuppressive and/or antibiotic treatment prior to their inclusion in the study (azathioprine 2.5 mg/kg/day for at least 6 months, plus metronidazole 500 mg/12 h and ciprofloxacin 500 mg/12 h for at least 6 weeks). Infliximab treatment consisted of an i.v. infusion of 5 mg of infliximab per kilogram of body weight at weeks 0, 2, and 6.
Patients were followed up for at least 10 weeks from treatment onset in order to evaluate their clinical response. A clinical evaluation was carried out at weeks 0, 2, 4, 6, 8, and 10. An assessment of HRQOL was made at weeks 0, 4, and 10 using the validated SF-36 and IBDQ tests.
CLINICAL EVALUATION
Besides evaluating partial or complete fistula closure in order to correlate clinical activity and quality of life, we used the Crohn's disease activity index (CDAI), which has been previously used to evaluate the efficacy of infliximab in the treatment of CD (7). CDAI incorporates eight related variables:
1. Number of stools per day.
2. Severity of abdominal pain.
3. General well-being.
4. Extraintestinal manifestations.
5. Abdominal mass.
6. Use of antidiarrheal drugs.
7. Hematocrit.
8. Body weight (16).
These items yield a composite score ranging from 0 to 600; scores below 150 indicate remission, whereas scores above 400 indicate severe illness. A CDAI was calculated every two weeks over ten weeks of follow-up (weeks 0, 2, 4, 6, 8 and 10).
HEALTH RELATED QUALITY OF LIFE ASSESSMENT (HRQOL)
Health-related quality of life (HRQOL) was assessed by means of a generic questionnaire, the SF-36 Health Survey (SF-36), and by means of the disease-specific inflammatory bowel disease questionnaire (IBDQ), both previously translated into Spanish and validated (17). These HRQOL questionnaires were self-administered by patients at weeks 0, 4 and 10,
SF-36 is a generic HRQOL instrument which includes eight dimensions:
1. PF - physical functioning (10 questions).
2. SF - social functioning (2 questions).
3. PR - physical role (4 questions).
4. ER emotional role (3 questions).
5. MH - mental health (5 questions).
6. VT - vitality (4 questions).
7. BP - bodily pain (2 questions).
8. GH - general health (5 questions) and two summary scores (PCS - Physical Component Summary; and MCS - Mental Component Summary). Each dimension of SF-36 can be scored from 0 (worst HRQOL) to 100 (best HRQOL). A standardization of these scores was made according to age and gender using Spanish population normative data (18), which were obtained from a study carried out on a random stratified sample of 9,151 subjects from the general population who answered the questionnaire, and by applying a formula proposed by the authors (20), that is:
Standarized score = patient score - population mean
score/population standard deviation
Here "patient score" is the score for the patient (from 0 to 100). "Population mean score" is the mean score for a group from the Spanish general population of the same age (divided into groups of 10 years) and gender. "Population standard deviation" is the standard deviation corresponding to the mean score of the general population used. A standardized score greater than 0 indicates better HRQOL than the general population of same age and gender; and a score below 0 indicates worse HRQOL.
The Spanish version of IBDQ was validated from the 36-item version of Love et al (21). IBDQ items are group-ed into five domains of health:
1. Bowel symptoms (8 items).
2. Systemic symptoms (7 items).
3. Functional impairment (7 items).
4. Social impairment (6 items).
5. Emotional function (8 items).
Responses are scored on a seven-point Likert scale, in which 7 corresponds to the best function and 1 to the worst function.
STATISTICAL ANALYSIS
Differences between means were assessed using Mann-Whitney's test. Those between proportions were established by using Fisher's exact test. Paired median scores were compared using Wilcoxon's test. Correlations among IBDQ, SF-36 and CDAI scores were calculated using Spearman's correlation. The accepted level of statistical significance was considered as p < 0.05.
Results
Sample description
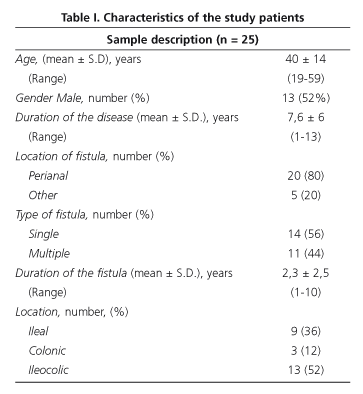
The characteristics of the patients studied are shown in table I. Most patients had a perianal fistula (80%) and 44% had multiple fistulas. In accordance with the Vienna classification of CD (15) all patients were diagnosed as having CD under forty years of age, and the major illness site was the ileocolonic tract (52%) (Table I).
Clinical response
Of 25 patients receiving infliximab, 16 (64%) responded to treatment, this being considered a complete closure of fistulas. Of these, 14 responded completely within the first 2 weeks.
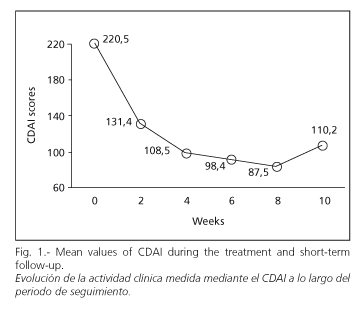
Regarding the CDAI index, total score decreased during treatment. Mean values at weeks 0, 4, 8 and 10 were 220.5, 108.5, 87.5 and 110.2, respectively (p < 0.01 between weeks 0 and 10) (Fig. 1).
Treatment was well tolerated and few secondary effects were observed during infusion (headaches, dizziness and fever in 2 patients) (8%).
HRQOL response
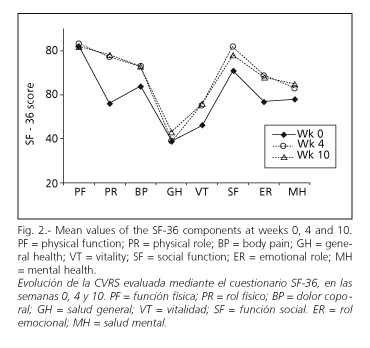
Mean standard scores on SF-36 increased in all cases when week 0 was compared to week 10. Differences between weeks 4 and 10 were not statistically significant in any of the cases (Fig. 2).
In the IBDQ test a significant increase was observed in total score on comparing week 0 to weeks 4 and 10 (p < 0.01). Between weeks 4 and 10 the difference in score did not reach statistical significance. The scores for the different scales, when studied separately, also showed a statistically significant difference in all cases (p < 0.01), except for social functioning (Fig. 3).
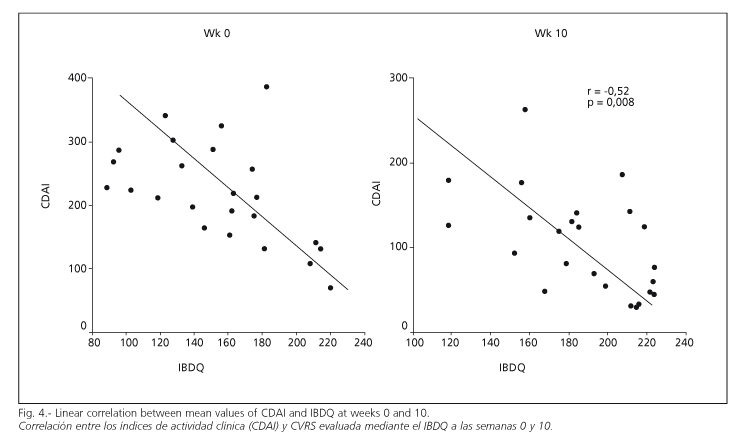
A correlation was observed between clinical response as measured by CDAI and improvement in quality of life as registered in the IBDQ questionnaire (Fig. 4) (r = - 0.57; p = 0.003), (r = - 0.53; p = 0.007) and (r = - 0.52; p = 0.008) at weeks 0, 4 and 10, respectively. This correlation could not be demonstrated between CDAI and SF-36 questionnaires, in neither the initial nor the final scores of both questionnaires (r = -0.084 and r = 0.106). This demonstrated the greater sensitivity of measuring quality of life using specific, rather than generic questionnaires (Fig. 4).
On analyzing the different components of SF-36, differences were statistically significant for physical role (PR), bodily pain (BP), general health (GH), vitality (VT), mental health (MH) and physical component summary (PCS) scores when week 0 was evaluated with respect to week 4, and for physical role (PR), social functioning (SF) and the physical component summary (PCS) (Fig. 5).
DISCUSSION
This prospective observational study describes the response to short-term treatment with infliximab and its effect on HRQOL in patients with fistulizing CD. This character of the study, without group manipulations, allows us to know the way this treatment behaves in clinical practice. Outside the realm of "clinical trials" infliximab is shown to be effective in the treatment of fistulizing CD, and improvements in HRQOL have been reported by patients.
The percentage response to treatment (64%) is similar to that reported by Present et al (68%) with the same dose of this drug (7). The measurement of response using CDAI also shows scores similar to those referred to by these authors, although in our study the initial mean score was greater, but the final result reached showed a similar mean. In this way a clear clinical response to infliximab can be observed in this group of patients.
HRQOL of patients treated with infliximab and evaluated using the IBDQ and SF-36 Health Questionnaires also shows significant improvement. Good tolerance to treatment should also be stressed, as there were few significant adverse effects and it was no therapy interruption or withdrawal was deemed necessary.
Other immunomodulatory drugs such as azathioprine (AZA) and 6-mercaptopurine (6-MP) have also been shown to be efficient for the improvement of HRQOL, as the number of relapses decreased (3). These drugs, however, have risks of toxicity, delayed action and need for monitorization, and their use has thus a lesser impact on health as perceived by the patient. Methotrexate (MTX), another effective immunosupressive agent, has also demonstrated a significant improvement in IBDQ scores, although at the expense of a high number of dropouts from treatment due to the development of undesirable secondary effects (22).
Generic questionnaires such as SF-36 have been shown to be useful in the evaluation of quality of life in patients with Crohn's disease (23). Our results show a significant improvement in quality of life as measured using this instrument, this being seen above all in physical aspects. Increases in PCS (physical component summary) are greater than those of MCS (mental component summary), and this has been shown to be statistically significant.
The impact of treatment with infliximab on the IBDQ scores is in accordance with that reported by Lichtenstein et al (14), although in this study measurements were made in the context of a clinical trial which included a placebo group, and the improvement of IBDQ was significantly greater in the therapeutic group versus the placebo group. The results of this study did not consider the subgroup of patients with fistulizing disease separately. Neither did the study by Present et al (7) analyze the influence of infliximab on quality of life in fistulizing CD -it only demonstrated its clinical efficacy.
The IBDQ questionnaire is the more specific, most extensive and most widely used questionnaire today in the assessment of quality of life using the various treatments for inflammatory bowel disease (24). It allows us to evaluate overall response both through and across its different domains. Our results show an improvement in overall score and in the scores of its different domains, except for social functioning. This result is similar to that reported in a study by Casellas et al (10), where the social dimension was less affected in these patients versus other dimensions such as the organic and emotional ones. In agreement with these authors this could be due to the fact that patients with CD attempt to maintain their social activities, even when the disease remains active. It has been suggested that patients with poorer social functioning in IBDQ may visit their gastroenterologists more often, and are also hospitalized with greater frequency, than those with better social functioning (25).
The considerable effect that fistulizing disease has on quality of life makes its measurement necessary in the evaluation of the different treatments available for the disease. Perhaps the best option is IBDQ, given its high sensitivity and good validation in many studies.
Further quality of life findings are expected from ongoing clinical trials of infliximab long-term maintenance treatment in patients with fistulizing CD.
REFERENCES
1. Lichtenstein GR. Treatment of fistulizing Crohn's disease. Gastroenterology 2000; 119: 1132-47. [ Links ]
2. Williams DR, Collier JA, Corman ML, Nugent FW, Veidenheimer MC. Anal complications in Crohn's disease. Dis Colon Rectum 1981; 24: 22-4. [ Links ]
3. Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn's disease: a meta-analysis. Ann Intern Med 1995; 123: 132-42. [ Links ]
4. Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Parternack BS. Treatment of Crohn's disease with 6 mercaptopurine: a long term, randomized, double-blind study. N Eng J Med 1980; 302: 981-7. [ Links ]
5. Hanauer SB, Smith MB. Rapid closure of Crohn's disease fistulas with continuous intravenous cyclosporin A. Am J Gastroenterol 1993; 88: 646-9. [ Links ]
6. Bell S, Kiamm MA. Antibodies to tumour necrosis factor µ as treatment for Crohn's disease. Lancet 2000; 355: 858-60. [ Links ]
7. Present DH, Rutgeerts PR, Targan S, Hanauer SB, Mayer L, Hogezand V, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999; 340: 1398-405. [ Links ]
8. Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Gastroenterology 1994; 106: 287-96. [ Links ]
9. Bungay KM,Ware JE. Medición y control de la calidad de vida relacionada con la salud, conceptos actuales. Monografía editada por Upjohn Company, Kalamazoo, Michigan. 1996. [ Links ]
10. Casellas F, Lopez Vivancos J, Badia X, Vilaseca J, Malagelada JR. Influence of inflammatory bowel disease on different dimensions of quality of life. Eur J Gastroenterol Hepatol 2001; 13: 567-72. [ Links ]
11. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989; 96: 804-10. [ Links ]
12. Targan Sr, Hanauer SB, van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor µ for Crohn's disease. N Eng J Med 1997; 337: 1029-35. [ Links ]
13. Rutgeerts P, D'Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology 1999; 117: 761-9. [ Links ]
14. Lichtenstein GR, Bala M, Chenglong H, De Woody K, Schaible T. Infliximab improves quality of life in patients with Crohn's disease. Inflamm Bowel Dis 2002; 8: 237-43. [ Links ]
15. Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn's disease: Report of the Working Party for the World Congress of Gastroenterology, Vienna 1998. Inflamm Bowel Dis 2000; 6: 8-15. [ Links ]
16. Best WR, Becktel JM, Singleton JW, Dern FJr. Development of a Crohn's disease activity index: National Cooperative Crohn's Disease Study. Gastroenterology 1976; 70: 439-44. [ Links ]
17. Alonso J, Prieto L, Antó JM. La versión española del SF-36 Health Survey (Cuestionario de Salud SF-36): Un instrumento para la medida de los resultados clínicos. Med Clin-Barcelona 1995; 104 (20): 771-6. [ Links ]
18. Alonso J, Regidor E, Barrio G, Prieto L, Rodríguez C, de la Fuente L. Valores poblacionales de Referencia de la versión española del Cuestionario de Salud SF-36. Med Clin-Barcelona 1998; 111 (11): 410-6. [ Links ]
19. Ware JE, Konsinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales:a user's manual. The Health Institute, New England Medical Center, Boston, Massachusetts, 1994. [ Links ]
20. López-Vivancos J, Casellas F, Badia X, Vilaseca J, Malagelada JR. Validation of the Spanish version of the inflammatory bowel disease questionnaire in ulcerative colitis and Crohn's disease. Digestion 1999; 60: 274-80. [ Links ]
21. Love JR, Irvine EJ, Fedorak RN. Quality of life in inflammatory bowel disease. J Clin Gastroenterol 1992; 14: 15-9. [ Links ]
22. Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn's disease. N Engl J Med 1995; 332: 292-7. [ Links ]
23. Blondel-Kucharski F, Chircop C, Marquis P, Cortot A, Baron F, Gendre JP, et al. Health-related quality of life in Crohn's disease: a prospective longitudinal study in 231 patients. Am J Gastroenterol 2001; 96: 2915-20. [ Links ]
24. Casellas F, Lopez-Vivancos J, Vergara M, Malagelada JR. Impact of inflammatory bowel disease on health-related quality of life. Dig Dis 1999; 17: 208-18. [ Links ]
25. De Boer A, Wijker W, Bartelsman J, de Haes HCJM. Inflammatory bowel disease questionnaire: cross-cultural adaptation and further validation. Eur J Gastroenterol Hepatol 1995; 7: 1043-50. [ Links ]











 texto en
texto en