Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.7 Madrid jul. 2004
| PICTURES IN DIGESTIVE PATHOLOGY |
Apoptosis: a rapid and silent form of death
J. A. Solís Herruzo
Department of Gastroenterology. Hospital Universitario 12 de Octubre. Madrid. Spain
Cells die by two different mechanisms: a) necrosis, by which the release of intracellular proteolytic enzymes induces tissue damage and inflammatory response; or b) apoptosis (Greek meaning apo, off; ptosis, falling), where the cell remnants quietly disappear as they are phagocytosed by surrounding cells. The latter, first described, in 1972, by Kerr et al. (1), is the process by which cells are removed under normal conditions when they reach the end of their life span, are damaged or are superfluous. It is estimated that normal apoptotic cell removal take place at a rate of 1x1011 cell per day, which is equivalent to the turnover of an adult's total body weight every 18-24 months.
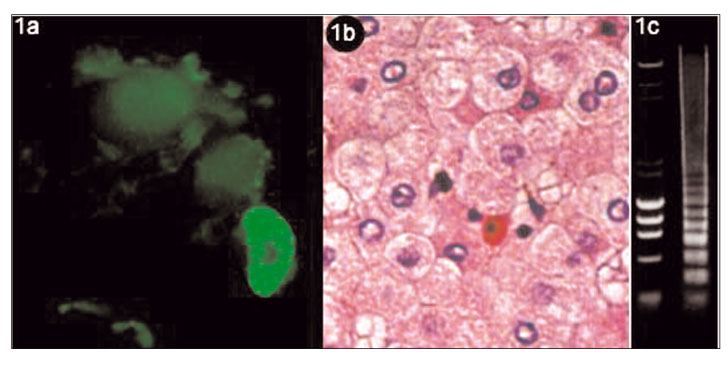
In cells undergoing apoptosis, there is shrinkage, lost of specialized surface features (microvilli), ruffling, membrane blebbing, condensation and margination of nuclear chromatin and nuclear fragmentation (Fig. 1a). Afterwards, there is cellular fragmentation into membrane-enclosed "apoptotic bodies" that are engulfed rapidly by tissue macrophages and neighboring cells. This process is typically complete in only 30 to 60 minutes. As no cytosolic contents are released during apoptosis, inflammation is not triggered. The microscopic appearance of an apoptotic cell consists of intense eosinophilic cytoplasm, condensed chromatin masses and picnotic nucleus (2). Councilman bodies (Fig. 1b), a well known pathological feature of hepatocellular death, represent apoptotic bodies. In the final stage of apoptosis, endonucleases cleavage DNA in the internucleosomal linker regions giving rise to about 200-base-pair fragments. Separation of these fragments by agarose gel electrophoresis reveals the characteristic DNA ladder pattern of apoptosis (Fig. 1c), which is in contrast to the smudge pattern seen with cell necrosis caused by the fully degraded DNA.
Apoptosis is an integral part of normal tissue development and homeostasis, eliminating redundant cells during embryogenesis. Furthermore, apoptotic cell death can be induced by multiple stimuli, including oxidative stress, radiation, absence of growth factors, and exposition to chemotherapeutic agents, transforming growth factor ß, Fas/Fas ligand system or tumor necrosis factor α (3). These factors fire the enzyme system responsible for an ATP-dependent cell death called caspase cascade (c-asp-ase: c-, cysteine; -asp, aspartic acid; ase, protease) (4). These highly conserved family of proteases "cut" cytoplasmic and nuclear content for packing in apoptotic bodies. Proteolysis by caspases is restricted to specific aspartic acid residues, producing disassembling of proteins, but not general proteolysis. A group of caspases are located close to the plasma membrane ("initiator caspases") and are upstream activators of the other group of caspases ("effector caspases"), which degrade cytoskeletal proteins, lamins (nuclear protein), poly-(adenosine-diphosphate-ribose) polymerase (DNA repair), histones, ribonuclear proteins, and nucleolins, among others, and activate caspase-activated DNAase (cleavage of DNA). Cleavage fragments of DNA and histone proteins are called oligonucleosomes, which contain about 200 base pair or integral multiples of 200 base pairs. This cleavage pattern is responsible for the characteristic DNA laddering seen in agarose gels.
REFERENCES
1. Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biology phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239-57.
2. Allen RT, Hunter WJ III, Agrawal DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Meth 1997; 37: 215-28.
3. Salvesen GS, Dixit VM. Caspase activation: The induced-proximity model. Proc Nat Acad Sci USA 1999; 96: 10964-7.
4. Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science 1998; 281: 1312-6.
5. Yu J, Zhang L. Apoptosis in human cancer cells. Curr Opin Oncol 2003; 16: 19-24.











 texto en
texto en