Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.96 no.10 Madrid oct. 2004
| CLINICAL NOTE |
Imatinib and gastrointestinal stromal tumor (GIST): a selective targeted therapy
A. Fernández and J. Aparicio1
Department of Gastroenterology and Hepatology. 1Department of Medical Oncology. University Hospital La Fe. Valencia, Spain
ABSTRACT
Gastrointestinal stromal tumors are the most frequent mesenchymal tumors in the gastrointestinal tract. They originate from the interstitial cells of Cajal and are characterized by an anomalous receptor for a growth factor with tyrosine-kinase activity (c-kit). This anomaly causes a permanent activation of the receptor and uncontrolled cell growth. These tumors show a poor response to traditional chemotherapy drugs, and are thus associated with low survival in cases of advanced disease. Imatinib, a tyrosine kinase inhibitor, is an example of selective targeted oncologic therapy that induces improved survival in these patients. We discuss two cases of metastatic gastrointestinal stromal tumors with a good response to imatinib, and also review the pathophysiology and treatment-related outcome of this type of tumors. We include results from clinical phase-III studies.
Key words: Gastrointestinal stromal tumor. Imatinib mesylate. Receptor protein-tyrosin kinase. C-kit.
Fernández A, Aparicio J. Imatinib and gastrointestinal stromal tumor (gist): a selective targeted therapy. Rev Esp Enferm Dig 2003; 95: 723-729.
Recibido: 25-09-03.
Aceptado: 15-12-03.
Correspondencia: Alberto Fernández Villaverde. Servicio de Medicina Digestiva. Hospital Universitario La Fe. Avenida Campanar, 21. 46009 Valencia. Tel.: 963 877 118. e-mail: afvillaverde@hotmail.com
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most frequent mesenchymal tumors in the gastrointestinal tract. They arise from the interstitial cells of Cajal at the myenteric plexus of the gut, which coordinate slow-wave peristalsis and act as gastrointestinal pacemaker cells. Their incidence ranges from 10 cases per million people and year in the United Kingdom to 4 cases in Finland, although these figures are probably underestimated (they represent 20-30% of all cases of soft-tissue sarcomas) (1,2). Most of them occur in the stomach (60-70%), small intestine (25-35%), colon and rectum (5%), and oesophagus (2%). Extra-gastrointestinal locations are very unusual, representing only 5-7% (3,4). GISTs usually develop in patients over 40 years of age, with a median age between 55 and 65 years, and are exceptional in children. There are no differences in sex distribution (5,6). Clinical presentation is variable, and the most common symptoms are diffuse abdominal pain, gastrointestinal bleeding, fever, and a palpable mass or even obstruction. Sometimes they are incidental findings during abdominal surgery or gastroscopy in the form of submucosal nodules. Confirmatory diagnosis is based on immunohistochemical features such as those of a highly cellular, spindle cell or epitheloid mesenchymal tumor that expresses the kit protein (7).
GISTs are a relatively recent diagnostic group of tumors; they arise from the classic leiomyosarcomas of the gut as a group of sarcomas with different immunohistochemical features. In 1994 it was demonstrated that these tumors had CD-34 as a cell marker, which allowed separating GISTs from true smooth-muscle tumors and schwannomas (8,9). Subsequently, it was discovered that the interstitial cells of Cajal expressed a receptor for a stem-cell factor with tyrosine kinase activity known as c-kit. The interaction factor-receptor relays intracellular signals (2,10) that alter gene transcription, cell proliferation, and apoptosis. In 1998 the majority of malignant GISTs were shown to have DNA mutations that expressed a mutated form of c-kit, one which was permanently activated even without ligand. This continuous activation leads to uncontrolled growth cell and an abolition of apoptosis, and is regarded as the main cause of GIST. Ninety-five percent of these tumors stained positively with a polyclonal c-kit antibody (CD117), and this is used as the gold standard for immunohistochemical diagnosis (11). The recognition of this molecular mechanism allowed the synthesis of selective protein-tyrosine kinase inhibitors, a specific treatment for a molecular target.
We report two cases of GIST treated with imatinib, a tyrosine kinase inhibitor.
CASE REPORT 1
A 39-year-old woman was admitted to our hospital in September 2002 with a 24-hour history of biliary vomiting, and 2-month-standing dull epigastric pain with abdominal distension, constitutional syndrome, asthenia, anorexia, and non-measured weight loss. Physical examination revealed a good nutritional status and a palpable painful mass in the upper quadrant of the abdomen above 8 cm in diameter. Lymph nodes were not enlarged.
Laboratory tests revealed a white blood cell count of 19,100/ml with neutrophilia, 483,000 platelets/ml, serum fibrinogen of 596 mg/dl, and lactic dehydrogenase at 1201 UI/l. Plain chest x-rays showed no alterations; plain abdominal x-rays revealed evidence of a mass in the left abdomen. An esophagogastroscopy was performed, which showed a 4 cm mass in the greater curvature of the stomach. Biopsies were taken, and no malignancy signs were revealed by the histological evaluation. An abdominal contrast CT scan was carried out, which showed numerous hypodense lesions in both hepatic lobes, with a greater diameter of 14 x 8.5 cm, and an irregular mass adjacent to the stomach (Fig. 1). On the basis of these findings, a biopsy of one liver lesion was performed. Histological examination of the biopsy specimen showed a solid proliferation of small spindle-like cells with atypical nuclei over a smooth mixoid stroma. These cells were mesenchymal (vimentin-positive) and grouped in bundles, rosetoid formations, or trabecullated patterns, with occasional mitoses. A diagnosis was made of low-grade, fusocellular sarcoma with CD117 positive staining.
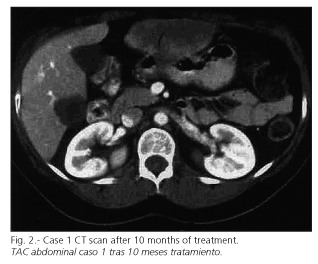
Surgical resection was ruled out because of the liver metastases, and the patient started treatment with oral imatinib (STI 571), initially 400 mg per day, and then titrated to 800 mg daily with good tolerance. The only adverse event observed was periorbital edema. Clinical response was obvious with a clear reduction in tumor bulk within 3 months, and normal performance status. Consecutive CT scans showed a progressive reduction in both size and density of both gastric and liver lesions. The patient is presently under regular follow-up and asymptomatic. After 10 months of treatment a new CT scan showed the most relevant liver lesion with a size of 8.5 x 5 cm, and the gastric lesion with a size of 4 x 3.5 cm (Fig. 2).
CASE REPORT 2
A 68-year-old woman was admitted to the emergency room in January 2002 with a few-week history of abdominal distension. A great abdominal mass was observed during physical examination. An abdominal CT contrast scan was performed, which showed a 20 cm heterogeneous mass adjacent to the spleen, stomach and small bowel, and related to the liver, uterus and ovaries. An open surgical procedure showed a mass occupying the omental bursa with both solid and hematic contents. A tumorectomy and partial gastrectomy were performed. The histological evaluation of the specimen revealed a malignant gastrointestinal stromal tumor (GIST).
Follow-up examinations were uneventful until July 2002, when a new abdominal distension was observed. A CT scan was performed (Fig. 3), which showed a mesenteric tumor composed of numerous heterogeneous and rounded lesions with necrotic areas inside throughout the peritoneum. There were also metastases in the left hepatic lobe, and evidence of peritoneal spreading. The patient started treatment with imatinib orally, 400 mg per day. A higher dose was not given because of the development of adverse events: facial edema, vomiting, and dry mouth. Four months later a new CT scan was performed, and liver lesions had disappeared whereas peritoneal spreading had reduced its surface clearly, as can be seen in figure 4. Clinical evolution was favorable, with a normal performance status and no complications seen in follow-up examinations. The last CT scan was performed in March 2003, and no new lesions were found.
DISCUSSION
Complete surgical resection is the treatment of choice for localized GISTs. However, surgery is rarely curative because of the trend of this tumor to metastasize and spread intra-abdominally. Even low-grade malignant tumors (< 50 mitoses per high-power field) often develop metastases 10 to 15 years after primary surgical resection. Furthermore, about 50% of patients present with metastases at the time of diagnosis. Thus, overall 5-year survival ranges between 28 and 35% for completely or partially resected tumors, and the best result obtained is around 54% in completely resected tumors (12). The median duration of survival for patients with metastatic disease is approximately 20 months, and 9 to 12 months for local recurrence. Standard chemotherapy and/or radiation therapy are of limited efficacy: doxorubicin- and ifosfamide-containing regimens have reported response rates of 0 to 27%, with 0 to 7% for paclitaxel and gemcitabine, respectively (13).
However, the prognosis of unresectable or metastatic GISTs has dramatically changed with the development of imatinib, a selective tyrosine kinase inhibitor. This drug is a specific molecular treatment that competes for the ATP-binding site of tyrosine kinase receptor and blocks the intracellular signaling pathways responsible for uncontrolled growth cell. Imatinib is an example of selective targeted oncologic therapy. The first reported trial of this drug was in 1996 (14) in the treatment of chronic myeloid leukemia (Bcr-Abl tyrosine kinase was identified as an abnormal molecular target). The first GIST treated with imatinib was reported in 2000 (15). Because of this successful initial case, several trials were performed. We would like to emphasise the multicenter study by Demetri et al. (16) in 2002: 147 patients with advanced GIST were randomly assigned to receive either 400 or 600 mg per day of imatinib. In all, 53.7% of patients had a partial response (reduction in the bulk of the tumor from 50 to 96%) and the one-year actuarial disease-specific survival was 88%. Similar results were reported in different trials (17).
In order to confirm these results and to assess the appropriate dose of imatinib, two randomized phase-III trials were initiated in December 2001 in the United States, Europe and Canada. Preliminary data from the US/Canadian trial were recently reported (18). In all, 647 patients with GIST were included and were randomly assigned to receive either 400 to 800 mg daily; those who developed progressive disease on the 400 mg/d group were titrated to 800 mg. No significant differences in survival were found. The one-year progression-free survival was 71% for the first group and 70% for the 800 mg/d group, and one-year overall survival was 86 and 85%, respectively. The incidence of toxicity was significantly higher in the second group. Normal hemoglobin levels, good performance status, and a gastric origin of metastases were identified as factors predictive of response (19).
Imatinib is given orally, and usual dosage ranges from 400 to 1.200 mg per day. This treatment is usually well tolerated, and there has been no evidence of tumor lysis syndrome. Most frequent adverse events include periorbital or facial edema (in 75% of patients, and correlated with low albumin levels), but these revert by diminishing the dose. Also nausea (52%), diarrhea (50%), myalgia, dermatitis, headache, and abdominal pain have been described (15). Hematological toxicity is exceptional and correlates with low hemoglobin rates. Fever or anorexia may develop at the beginning of treatment, but usually disappear spontaneously in a few days. Cases of serious intratumoral hemorrhage that may occasionally need urgent surgical treatment have been reported; thus closer follow-up examinations are recommended.
Imatinib is considered the drug of choice for advanced GIST. However, several clinical challenges remain. The complete response rate appears to be low. Longer follow-up and further studies are necessary to determine the influence of imatinib on long-term survival, as well as its curative potential. Also, we do not know its benefit as adjuvant therapy after complete surgical resection. The appearance of resistance to imatinib after long lasting treatment is high: the median duration of response to this drug is 17 months. Four different mechanisms of resistance have been reported: acquisition of new mutations in kit, overexpression of c-kit, activation of an alternate tyrosine kinase receptor without kit expression (platelet-derived growth factor receptor-alpha), and other genomic mutations (20-22). Several studies are scheduled to resolve these clinical problems: a new drug called SU11248 showed efficacy on imatinib-resistant GISTs in a small clinical trial (23). Furthermore, not all the factors related to low response or resistance have been identified as yet.
In conclusion, we would like to emphasise the importance of performing biopsies and a CD-117 study for all submucosal or stromal gastrointestinal tumors, even in metastatic cases and whatever the size. If we diagnose a gastrointestinal stromal tumor, even in non-resectable cases we can offer an important improvement in survival and performance status with minimum adverse events by using this selective tyrosine-kinase inhibitor (imatinib).
REFERENCES
1. Roberts J, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer 2002; 38 (Supl. 5): S37-8. [ Links ]
2. Kindblom L-G, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the intersticial cells of Cajal. Am J Pathol 1998; 152: 1259-69. [ Links ]
3. Emory TS, Sobn LH, Lukes L, et al. Prognosis of gastrointestinal sooth-muscle (stromal) tumors. Am J Surg Pathol 1999; 23: 82-7. [ Links ]
4. Martínez-Rodenas F, Pie J, Gómez M, et al. Extra-gastrointestinal stromal tumour-semiology abd clinical therapy peculiarities. Rev Esp Enferm Dig 2002; 94: 625-32. [ Links ]
5. Judson I. Gastrointestinal stromal tumours (GIST): biology and treatment. Ann Oncol 2002 ; 13 (Supl. 4): 287-9. [ Links ]
6. Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer 2002; 38 (Supl. 5): S39-51. [ Links ]
7. Miettinen M, Lasota J. Gastrointestinal stromal tumors: definition,clinical, histological and molecular genetic features and differential diagnosis. Virchows Arch 2001; 438: 1-12. [ Links ]
8. Monihan JM, Carr NJ, Sobin LH. CD34 immunoexpresion in stromal tumours of the gastrointestinal tract and in mesenteric fibromatosis. Histopathology 1994; 25: 469-73. [ Links ]
9. Miettinen M, Virolainen M, Sarlomo-Rikala M. Gastro-intestinal stromal tumors: value of CD34 antigen in their identification and separation from the leiomyomas and schwannomas. Am J Surg Pathol 1995; 19: 207-16. [ Links ]
10. Vliagofris H,Worobec AC, Mercalfe DD. The proto-oncogene c-kit ligand in human disease. J Allerg Clin Immunol 1997; 100: 435-40. [ Links ]
11. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279: 577-80. [ Links ]
12. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors. Recurrence patterns and prognostic factors for survival. Ann Surg 2000; 231: 51-8. [ Links ]
13. DeMatteo RP, Heinrich MC, El-Rifai WM, et al. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol 2002; 33: 466-77. [ Links ]
14. Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the ABL tyrosine kinase on the growth of BCR-ABL positive cells. Nature Med 1996; 2: 561-6. [ Links ]
15. Joensuu H, Roberts PJ, Sarlomo-Rikala, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastasic gastrointestinal stromal tumor. N Engl J Med 2001; 344: 1052-6. [ Links ]
16. Demetri G, von Mehren M, Blanke C, et al. Efficacy and safety of imatinib mesylate in avanced gastrointestinal stromal tumors. N Engl J Med 2002; 347: 472-80. [ Links ]
17. Van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastasic gastrointestinal tumours: a phase I study. Lancet 2001; 358: 1421-3. [ Links ]
18. Patel R. Early results from randomized phase III trial of imatinib mesylate for gastrointestinal stromal tumors. Curr Oncol Rep 2003; 5: 273. [ Links ]
19. Van Glabbeke M, Verweij J, Casali P, et al. Prognostic factors of toxicity and efficacy in patients with Gastro-intestinal Stromal Tumors (GIST) treated with imatinib: a studyof the EORTC-STBSG, ISG and AGITG. (abstract). Proc ASCO, 2003. [ Links ]
20. Fletcher J, Corless C, Dimitrijevic S, et al. Mechanisms of resistance to Imatinib Mesylate in advanced gastrointestinal stromal tumor. (abstract) Proc ASCO, 2003. [ Links ]
21. Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutation in the gastrointestinal stromal tumors. Science 2003; 299: 708-10. [ Links ]
22. Bernet L, Bustamante M, Zuñiga A, Cano R. Characterization of GIST/GIPACT tumors by immunohistochemistry and exon 11 analysis of c-kit gene by PCR. Rev Esp Enferm Dig 2003; 95: 688-91. [ Links ]
23. Von Mehren M. Recents advances in the management of gastrointestinal stromal tumors. Curr Oncol Rep 2003; 5: 288-94. [ Links ]











 texto en
texto en