Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.97 no.5 Madrid may. 2005
| ORIGINAL PAPERS |
Treatment with pegylated interferon alpha 2b and ribavirin in patients unresponsive to previous treatments with standard interferon as monotherapy or combined with ribavirin
F. Carnicer1, P. Zapater2, A. Gutiérrez3, A. García4, F. Ruiz5, M. López6, and Grupo de Estudio de Hepatitis de la Provincia de Alicante (GEHPA)
1Hepatic Unit. 2Service of Pharmacology Clinical. Hospital General Universitario de Alicante, 3Hospital General Universitario de Elche.
4Hospital de Villajoyosa. 5Hospital General Universitario de San Juan. 6Hospital de Alcoy. Alicante, Spain
ABSTRACT
Background: little information is available on the effect of pegylated interferon (PEG) and ribavirin (RBV) in patients with chronic hepatitis due to virus C (CHC) who were non-responders to previous treatment.
Objectives: to evaluate response to treatment in patients who were non-responders to previous treatment.
Methods: one hundred and twenty-four patients who were non-responders to previous treatment were included. All patients were treated with PEG alpha 2b interferon (dose: 1.5 mg/kg body weight) and RBV (weight-dependent dosage). A qualitative PCR of virus C after six months was evaluated. In those in whom this was positive, treatment was discontinued; in those who were negative treatment was continued to the end of the year.
Results: response following treatment (RFT) was 35.4% (44 patients), and sustained viral response (SVR) 29.8% (37 patients). No relation was observed between RFT, SVR and any previous treatment. RFT was dependent on low initial viremia and SVR was significantly and independently related to low serum hepatitis C RNA and a non-1 genotype. In general, treatment was well tolerated. Medication was discontinued in 5 patients, and doses reduced in 18.
Conclusion: on retreatment with PEG and RBV a SVR of 29.8% was achieved in patients who had not responded to previous treatment, so its use in this group of patients is indicated.
Key words: Treatment. Peginterferon and ribavarin. Unresponsive to treatment. Hepatitis C.
Carnicer F, Zapater P, Gutiérrez A, García A, Ruiz F, López M, and Grupo de Estudio de Hepatitis de la Provincia de Alicante (GEHPA). Treatment with pegylated interferon alpha 2b and ribavirin in patients unresponsive to previous treatments with standard interferon as monotherapy or combined with ribavirin. Rev Esp Enferm Dig 2005; 97: 306-316.
GEHPA: S. Pascual1, J.M. Palazón1, M. Pérez-Mateo1, S. Such1, M. F. García3, E. Girona3, R. Laveda3, J. Valverde4, C. Quílez4, A. Martínez5, F. de Vera6
This study has been realized, in part, by a grant of Instituto de Salud Carlos III, Madrid (CO3/02).
Recibido: 13-07-04.
Aceptado: 30-11-04.
Correspondencia: Fernando Carnicer. Unidad Hepática. Hospital General Universitario. C/ Pintor Baeza, s/n. 03010 Alicante. e-mail: canicer_fer@gva.es
INTRODUCTION
The efficacy of treatment in patients with chronic hepatitis due to virus C (CHC) has improved considerably since pegylated interferon (PEG) was introduced, as has been shown by the results of clinical trials (1,2). Currently the treatment of choice for CHC is PEG together with ribavirin (RBV). This is especially effective in patients who are being treated for the first time.
Little information is available regarding the results of treatment with PEG and RBV in patients who did not respond to previous treatment with interferon (IFN) as monotherapy or combined with RBV. A high rate of sustained viral response to IFN and RBV has been reported in relapsing patients who were previously given monotherapy with standard IFN (3), whilst results in unresponsive patients were less encouraging (4). However, it is interesting to note that re-treatment of unresponsive patients with PEG and RBV showed better results when standard combined treatment was used (5).
We therefore studied patients who had not responded to -or had relapsed after- standard IFN in monotherapy or combined with RBV to determine the efficacy of treatment using PEG and RBV in this group of patients.
PATIENTS AND METHODS
Selection of patients
We included patients diagnosed with CHC and previously given IFN as monotherapy or combined with RBV. Inclusion criteria included: a) patients diagnosed as having CHC and treated previously; and b) those with raised transaminases. The criteria for the diagnosis of CHC were: compatible liver biopsy, transaminase levels raised for > 6 months, and hepatitis C virus (HCV) RNA found. Patients were divided into two groups: a) non-responders: patients diagnosed as having CHC who had been given standard IFN and RBV for at least 6 months with no negative results being obtained when tested for virus C RNA; b) relapses: patients who had shown a good response following treatment (RFT) but in whom virus C RNA was again positive after treatment, i. e., patients without sustained viral response (SVR). A second biopsy was not essential for inclusion in the trial. There were intervals of 6 months to 3 years between failed previous treatment and current treatment.
Patients were excluded from the trial if they had anemia < 12 g/dl in women and 13 g/dl in men, neutropenia < 1500/mm3, thrombocytopenia < 90,000/mm3, serum creatinine > 1.5 times the normal value, co-infection with HIV, normal transaminases, active alcoholism or use of IV drugs during the previous 12 months, uncompensated cirrhosis, co-existent disease contraindicating treatment, and patients who had experienced severe adverse effects with previous treatment.
STUDY DESIGN AND ORGANIZATION
Before treatment was started we performed a full physical exploration, routine laboratory tests, coagulation studies, a PCR determination of HCV RNA, a genotype and a viral load using usual laboratory methods. Viremia was determined using a Roche Amplicor Monitor 2000 version, and genotype using a Versant HCV Genotype Bayer. Five hospitals in the Alicante province participated in a prospective observational study from June 2001 to June 2003. All information was obtained from clinical records and made anonymous for subsequent analysis. A total of 124 patients, routinely followed-up in the Gastro-intestinal Outpatients Department of these hospitals, were included in the study. These were divided into three groups: group I - 55 patients who had not responded to IFN monotherapy (44.8%); group II - 35 patients who had not responded to combined treatment (28.2%); group III - 14 patients who had relapsed after interferon treatment (11.3%) and 20 patients who had relapsed after combined treatment (16.1%).
Patients were treated with 1.5 mg/kg PEG 2b and RBV according to body weight. PEG 2b was given subcutaneously once a week throughout the established period. RBV was given orally every 12 hours. Adherence to treatment protocol was confirmed by questioning patients at every consultation.
Efficacy assessment
Six months after treatment onset the persistence of HCV RNA was determined using a qualitative PCR analysis. If this was positive, treatment was discontinued. Patients were considered RFT if the qualitative PCR was negative when treatment was complete. They were considered SVR when six months after treatment completion the qualitative PCR remained negative. All patients were followed up for at least six months after treatment completion.
We studied the predictive value of various parameters such as body weight, age, viremia, virus C genotype, transaminase levels, type of response to previous treatment, and type of previous treatment (standard IFN in monotherapy or combined with RBV) regarding response to treatment.
Safety assessment
Follow-up consultations took place at 2, 4, 6 and 8 weeks after treatment onset, and then every month until treatment completion, and subsequently at 60 and 72 weeks. When side effects occurred, the dose was corrected for both PEG and RBV in accordance with the technical data supplied with these drugs. If the cause of dose reduction resolved, the original dose was resumed.
Statistical evaluation
Quantitative data are presented as mean values and intervals between minimum and maximum values, and qualitative data as percentages or frequency. Differences between qualitative variables were calculated using an ANOVA test and the Bonferroni test for multiple comparisons. A Chi squared test was used to compare qualitative variables. A value of p < 0.05 was considered significant. The influence of the various variables on response to treatment for all patients studied was analyzed using an initial univariate logistic regression analysis. Variables showing a correlation of less than 0.2 in significance on the univariate analysis were subsequently included in a multivariate logistic regression analysis to detect factors predicting response to treatment.
RESULTS
Demographics
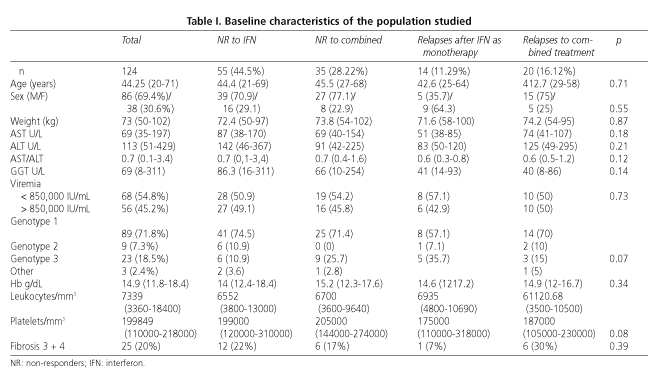
We studied 124 patients between June 2001 and June 2003; 86 (69.4%) were men and 38 (30.6%) were women, with an average age of 44 years (25-68). Their baseline characteristics are shown in Table I. There were no significant differences between groups or between patients from the various hospitals involved. No patients were lost to follow-up. Genotype distribution was similar in all groups, with a higher prevalence of genotype 1; this was 70% for all groups except group III, where it was 57%.
Viral response and RVS predictive factors
The analysis of response in 124 patients was RFT in 44 (35.4%) and SVR in 37 (29.8%).
A similar proportion of patients showed RFT in the 4 study groups: group I - 31%, group II - 40%, group III - 36%, group IV - 44% (p = 0.74). The percentage of patients with fibrosis of 3 or more was 20% in those with RFT and 23% in those who did not attain RFT (p = 0.69). Similarly, differences in gender distribution (73 vs. 66%; p = 0.46) and genotype 1 distribution (64 vs. 79%; p = 0.08) were not statistically significant in patients with or without RFT. There were no statistical differences regarding age (43 ± 10 vs. 46 ± 10; p = 0.19), weight or laboratory findings between patients with or without RFT. In all, 44% of patients with low viremia (< 850,000 IU/ml) showed RFT as compared with 28% of patients with high viremia (> 850,000 IU/ml) (p = 0.084).
A total of 37 patients (29.8%) showed RFT, whilst the remaining 7 patients relapsed. There was a similar proportion of patients with SVR in all four groups: group I - 28.8%, group II - 27.3%, group III - 40.0%, group IV - 33.3% (p = 0.59). The proportion of patients with fibrosis of 3 or more was 17.6% in those who responded and 23% in those who did not (p = 0.64). Nor was there any gender difference between those who responded and those who did not (73.5 vs. 65,7%; p = 0.72).
The groups of patients who responded, those who did not respond, and those who relapsed had significantly different proportions of genotype 1 distribution (58.8, 79 and 100%; p = 0.033), with a significantly lower (24%) proportion of SVR in patients with genotype 1 versus patients with genotypes 2-3 (45%) (p = 0.03), especially in group II, where 6 of the 9 patients who responded had genotype 3. There were no significant differences regarding age (43 ± 9 vs. 46 ± 10; p = 0.17), weight (72 ± 11 vs. 74 ± 11; p = 0.24) or laboratory parameters tested in patients with and without SVR. Regarding viremia, 41% of patients with low viremia (< 850,000 IU/ml) showed SVR when compared with 21% of those with raised viremia (> 850,000 IU/ml) (p = 0.088).
The logistic regression analysis (Table II) showed that only low viremia significantly correlated with an increased probability of RFT when factors such as age and genotype were considered. However, both low viremia and a genotype other than 1 are factors which are significantly and independently related to the probability of SVR.
Adverse effects
Side effects were very common but treatment only had to be discontinued in 6 patients (5%), and doses reduced in 22 patients (18%) (Table III).
Subjectively, patients considered that PEG-related side-effects were similar to those of standard IFN. They were mild and flu-like (asthenia, arthromyalgia and fever), and cutaneous lesions related to the site of PEG administration in 100% of cases. Average weight loss was 3.8 kg, and maximum weight loss was 16 kg. Major side effects included depression and personality changes. There was alopecia in 10% of patients. In two patients treatment was discontinued because of depression.
DISCUSSION
In our study, treatment with PEG-2b and RBV led to an RFT of 35.4% (44 patients) and an SVR of 29.8% (37 patients) in patients in whom previous treatment had not been effective. RFT was best in patients with genotypes 2-3, and SVR most marked in patients with genotypes other than 1 and in patients with a low viral load when treatment was begun, as previously reported (6-8). Disease relapsed in 7 patients (19%). There was no relationship between response and the remaining parameters studied, such as age, body weight and previous treatment.
Shiffmann et al. (9) recently reported on their study of patients who had failed to respond to previous treatment. In 18% of their patients they found SVR, which is lower than in our study. There may be several reasons for this discrepancy. Firstly they did a clinical trial whereas we made an observational study of our day-to-day clinical practice, which makes comparison difficult. The patient populations were also different. In addition, they studied a larger number of patients, and it is possible that if we had studied a larger group our results would have been more similar. In Shiffmann's study a higher proportion of patients had genotype 1 and high viremia. Both parameters significantly affect SVR. The peginterferon used was also different, since Shiffmann used peginterferon 2a whilst we used peginterferon 2b.
It has been said that relapsed patients respond better on further treatment than do patients who have not previously responded. However, we found a similar response rate in both groups of patients. This may be mainly due to the small number of patients in each group, making it difficult to find significant differences between them.
Side effects were similar to those seen in previously untreated patients. They were not serious and were generally well tolerated, so doses only had to be reduced in 18% and discontinued in 6 patients (5%).
The aim of this study was to show the result of CHC treatment in daily clinical practice as opposed to the experimental setting of a clinical trial. In this type of study, by its very nature, there is a risk of various types of bias. One problem is that data refer to a non-representative population of CHC patients. However, the scale of our study, in which all Gastrointestinal Departments at National Health hospitals in the health districts under study participated, means that only a few patients with CHC seen in other public health service departments and those treated privately were not included. There is no data to support that these patients would have responded differently.
Clinical trials are characterized by a strict control of the treatment given and the patients' response to it. In practice such control is not easy to achieve. In our study we used drug dosages similar to those used in clinical trials, as well as a similar frequency of follow-up consultations.
Patient adherence to the treatment protocol was assessed by direct questioning. This is a less reliable method than keeping a record of the medication given. However, in patients willing to accept a second course of treatment after putting up with side effects from a first course of similar treatment, the fact that they will take their medication reliably is more likely. Thus, it would seem that this willingness to accept a second course of treatment explains the small number of patients failing to complete it. Currently this aspect is considered to be very important, since it has been shown that dose reductions are directly related to reduced therapy efficacy (10).
In recent years there has been a great improvement in the response of CHC patients to treatment. Combined treatment with IFN and RBV (11), and especially the use of PEG has led to a marked improvement in response, particularly for genotype 1, with an overall response rate of over 50% in two large clinical trials (1,2). In spite of these advances many patients do not achieve SVR status. At present less information is available regarding response to treatment with PEG and RBV in patients who did not respond to previous treatment. Most data have been reported as communications in congresses, and it is not clear whether these patients were having "re-treatment" with PEG and RBV. In view of the increased efficacy of treatment in previously untreated patients, it would seem that the response of a similar group of patients who had not responded to treatment would show similar efficacy.
Before the advent of PEG, retreatment with standard IFN and RBV in patients who had not responded to monotherapy with IFN led to SVR in up to 50% of those with genotypes 2-3 who had relapsed (3,12). In patients who had not responded to combined treatment, the indication for retreatment with the same drugs was less clear, since results were worse with a response of 15% (4). With the introduction of PEG and RBV the situation appears to have changed, since there was an overall response rate of 30-50% on retreatment (5,6,13), with 18% for genotype 1 (5).
It has been possible to improve the response in relapsed patients by prolonging treatment for 12 months to obtain a 70% response rate (14). The result of using high doses of PEG in these patients is not yet clear. Diago et al. (15) obtained HCV RNA clearance after 12 weeks in previously non-responding patients with genotype 1, who were subsequently given double dosage of PEG 2a. Other authors do not report these results. Gross et al. (16) doubled the dose of PEG 2b and found no difference in response versus those who received usual dosage. It is reported that high doses of PEG 2b are well tolerated. With high dosage a larger proportion of patients failed to take their full doses, but complete interruption of treatment was similar in both groups. Similar results were obtained by White et al. (17), with improvement after 12 weeks but similar results after 24 weeks. In all studies high doses of PEG were well tolerated, but there was a greater percentage of patients in whom dosage was reduced amongst those taking high doses, although failure to complete the therapy course was similar for all groups. Induction doses have also been tried with no improvement in results (13).
In conclusion, our data, together with those of other authors, suggest that, in patients who have not responded to previous treatment, treatment with PEG and RBV is indicated, especially in those with genotype 2-3 and low viremia.
REFERENCES
1. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman ML, Reindollar R, et al. PEG alfa-2b plus ribavirin compared with INF alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958-65. [ Links ]
2. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. PEG alfa-2a plus ribavirin for patients with chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975-82. [ Links ]
3. Davis GL, Esteban R, Rustgi V, Hoefs J, Gordon SC, Trepo C, et al. INF alfa 2b alone o in combination withwith ribavirin for treatment of relapse of chronic hepatitis C. N. Engl J Med 1998; 339: 1493-9. [ Links ]
4. Diago M, Luján M, Valeros C, Tuset C, Marcaída G, García V, et al. Respuesta a largo plazo al INF y RBV en pacientes con hepatitis crónica C sin respuesta sostenida al INF. Rev Esp Enferm Dig 2001; 93: 353-8. [ Links ]
5. Gaglio PJ, Zimmerman D, Choi J, Heiler L, Brown RS. Treatment with pegylated INF alpha 2b and ribavirin (Rebetol) produces significant sustantained virologic response rates in HCV infected patients who failed prior therapy. Hepatology 2003; 38 (Supl. 1): 318A. [ Links ]
6. Minuk GY, Reddy KR, Lee S, Heathcote J, O'Grady J, Pockcros P, et al. Enhanced virologic response to treatment with 40KD PEG 2a in patients previously unresponsive to treatment with INF 2a. Hepatology 2001; 34 (Supl. 1): 330A. [ Links ]
7. Portal I, Botta-Fridlun D, Bourliere M, Rotily M, Halfon P, Couzigou P, et al. Treatment with pegylated INF alfa 2B in relapserto standard INF + ribavirin in chronic hepatitis C: Efficacy and safety results from a randomized multicentricfrenc study. Hepatoloy 2001; 38 (Supl. 1): 311A. [ Links ]
8. Adhal N, Flamm S, Malet PF, Tong M, Herrine SK, Brown R, et al. Analyses of 40 KDA PEG alfa 2a (Pegasys) in combination with ribavirin, mycophenolate mofetil, amantadine or amantadine lus ribavirin in patients that relapsed or did not respond to rebetron thetapy: a report of two randomized , multicenter, efficacy and safety studies. Hepatology 2001; 34 (Supl. 1): 243A. [ Links ]
9. Shiffman ML, Di Bisceglie, Karen AM, Lindsay L, Morishima C, Wrigh EC, et al. PEG Alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology 2004; 126: 1015-23. [ Links ]
10. McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 2002; 123: 1061-9. [ Links ]
11. McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. INF alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998; 339: 1485-92. [ Links ]
12. Camma C, Bruno S, Schepis F, Lo Iacono O, Andreone P, Gramenzi AJ, et al. Retreatment with INF plus ribavirin of chronic hepatitis C non-respondersto INF monotherapy: a meta-analisys of individual patientdata. Gut 2002; 51: 864-9. [ Links ]
13. Bapin C, Favor-Peray, Hachemane S, Blanc F, Díaz D, Puedo P, et al. Retratment with pegylated INF alpha 2b and ribavirin in patients with chronic hepatitis C non responders to INF monoterapy or INF and ribavirin combination. A prospective randomized pilote study of two regimens: Induction versus REG. J Hepatol 2004; 40 (Supl. 1): A135. [ Links ]
14. Di Marco V, Almasio PL, Vaccaro A, Ferraro D, Parisi P, Cataldo MG, et al. Combined treatment of relapse of chronic hepatitis C with high dose alfa 2b INF plus ribavirin for 6 or 12 months. J Hepatol 2000; 33: 456-62. [ Links ]
15. Diago M, Romero-Gómez M, Crespo J, Oliveira A, Pérez R, Bárcena R, et al. High-dose peginterferon alfa-2a (40 KD) (Pegasys®) and ribavirin (Copegus®)in patiens infected with hepatitis C virus (HCV) genotype 1 who failed to respond to interferon alfa and ribavirin. Hepatology 2003; 38 (Supl. 1): 740A. [ Links ]
16. Gross JB, Therneau TM, Johnson SM, Kwo PY, Afdhal NH, Flamm SL, et al. the renew trial: A national, multicenter study of high dose PEG alfa 2b + ribavirn for non-responders with hepatitis C. Hepatology 2003; 38 (Supl. 1): 312A. [ Links ]
17. White CL, Malet F, Wentworth CL, Catu VL, Alton TL, Lee WM. Interin results of a randomized trial of 1,5 µg/kg vs. 3,0 µg/kg pegylated interferon alfa 2b pegintron® (PEG) plus ribavirin (RBV) for naïve chronic hepatitis C patients and 3,0 µg/kg PEG plus RBV for non responders (NR) and relapsers (R) to previous therapy. (The target trial). Hepatology 2003; 38 (Supl. 1) 321A. [ Links ]











 texto en
texto en