Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Española de Enfermedades Digestivas
versión impresa ISSN 1130-0108
Rev. esp. enferm. dig. vol.101 no.3 Madrid mar. 2009
Predictive factors of poor response to intravenous cyclosporine in steroid-refractory ulcerative colitis
Factores predictivos de mala respuesta precoz a la ciclosporina endovenosa en la colitis ulcerosa grave corticorrefractaria
J. W. Huamán Ríos, F. Casellas Jordá and J. R. Malagelada Benaprés
Unitat d'Atenció Crohn-Colitis. Digestive System Research Unit. University Hospital Vall d'Hebron, Barcelona, Spain. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (Ciberehd)
ABSTRACT
Background: the treatment of severe ulcerative colitis (UC) flares includes measures such as hospitalization and intravenous steroids. Despite this, a quarter of patients are refractory to treatment. Given the availability of new therapeutic strategies in patients with steroid-refractory UC (cyclosporine, infliximab, apheresis, surgery) it is necessary to predict which treatment will be most effective for each patient.
Objectives: to determine which clinical or biological factors discriminate the lack of response to cyclosporine in steroid-refractory UC.
Methods: forty one flares of steroid-refractory UC in 35 patients treated with intravenous cyclosporine have been included. The response to cyclosporine was assessed at day 10 of treatment by using the modified Truelove and Witts disease activity score. Variables with prognostic significance were determined by a univariate analysis comparing groups with complete response and no-response, and an analysis of multiple linear regression.
Results: complete response was obtained in 41 flares (48%), partial response in 22%, and lack of response in 29%. The univariate analysis showed a significant difference in four predictive factors: higher age (p = 0.008), thrombocytosis (p = 0.01), disease extent (pancolitis vs. left-sided disease (p = 0.04)), and having received cyclosporine previously (p = 0.01). A multiple linear regression analysis confirmed the significance of higher age, thrombocytosis, and having received cyclosporine previously as predictive factors of poor response.
Conclusion: higher age, thrombocytosis and previous use of cyclosporine predispose to poor response to intravenous cyclosporine in severe flares of steroid-refractory UC.
Key words: Cyclosporine. Ulcerative colitis. Steroid refractory. Predictive factors.
RESUMEN
Introducción: el tratamiento de los brotes graves de colitis ulcerosa (CU) incluye medidas como la hospitalización y corticoides e.v., a pesar de ello, en una cuarta parte de los pacientes no se consigue inducir la remisión. Dada la disponibilidad de diferentes estrategias terapéuticas en los pacientes con CU corticorrefractaria (ciclosporina, infliximab, aféresis, cirugía) se hace necesario predecir que tratamiento será el más eficaz para cada paciente.
Objetivos: determinar de forma precoz qué factores clínicos o biológicos permiten discriminar los enfermos con CU corticorrefractaria que no responderán a ciclosporina.
Métodos: se han incluido 41 brotes de CU corticorrefractaria (35 pacientes) tratados con ciclosporina e.v. La respuesta se evaluó al día 10 de tratamiento con el índice de actividad de Truelove-Witts modificado. Para el análisis univariante se compararon los grupos con respuesta completa y mala respuesta. Las variables independientes con significación pronóstica se determinaron mediante análisis de regresión lineal múltiple.
Resultados: de los 41 brotes el 48% tuvieron una respuesta completa, el 22% tuvieron una respuesta parcial y el 29% tuvieron mala respuesta. El análisis univariante demostró diferencia significativa en cuatro factores predictivos: mayor edad (p = 0,008), trombocitosis (p = 0,01), extensión de la enfermedad (pancolitis versus colitis izquierda (p = 0,04)) y haber recibido ciclosporina previamente (p = 0,01). Sin embargo, en el análisis de regresión lineal múltiple no se confirmó la extensión de la enfermedad como factor predictivo de mala respuesta.
Conclusión: una mayor edad, la trombocitosis y el uso previo de ciclosporina predisponen a una mala respuesta a la ciclosporina endovenosa en los brotes graves de CU corticorrefractaria.
Palabras clave: Ciclosporina. Colitis ulcerosa. Corticorrefractaria. Factores predictivos.
Introduction
Ulcerative colitis (UC) is a chronic colonic immunoinflammatory disease characterized by periods of remission alternating with flares of variable activity with diarrhea, blood in stools, abdominal pain, and fever (1,2).
About 15% of patients with a UC flare-up will require admission to hospital and be treated with intravenous corticosteroids (3,4). Although intravenous corticosteroids are very effective, 20 to 47% of severe relapses need colectomy (5,6).
Since 1994 cyclosporine A (CyA) has been shown to be effective in reducing the need of colectomy in patients with severe UC refractory to corticosteroid treatment (7). However, 70% of patients who initially had a good response to CyA will require colectomy in the following 6 to 12 months (8). In addition, CyA treatment is related to frequent adverse effects, such as hypertension or renal insufficiency (9,10). Nowadays there are alternative strategies to CyA in severe relapses of UC refractory to corticosteroids, such as infliximab (11-16) or leukocytapheresis (17-19). Consequently, it is necessary to have easy indicators that allow to rapidly decide the therapeutic strategy in severe UC flares.
Several studies have established some predictors of severe UC non-respondent to intravenous corticosteroids: more than 8 stools/day, heart frequency higher than 100 pm, body temperature over 38 ºC, serum albumin lower to 3 mg/dl or lack of normalization of C-reactive pro-tein (20,21). However, there is scarce information relative to which factors can rapidly predict which patients with severe cortico-refractory UC will improve with CyA (22).
The main objective of the present study has been to rapidly determine which clinical or biological factors can identify patients with severe cortico-refractory UC that will not respond to CyA.
Material and methods
Patients
The clinical records of patients with severe UC treated with intravenous CyA admitted to Service of Digestive Diseases, University Hospital Vall d'Hebron, Barcelona (Spain), during the period 2000-2006 were reviewed. None of these patients responded to intravenous corticosteroids at a dose of 1 mg/kg during 1 week.
The diagnosis of UC was based on conventional clinical, endoscopical, and histological criteria (23-25).
UC severity was determined according to the modified clinical index by Truelove-Witts (mTLW) (26,27): scores of nine items were summed to obtain an index of remission (score lower to 11), mild activity (score from 11 to 15), moderate activity (score from 16 to 21), and severe activity (score between 22 and 27).
Procedure
Cyclosporine administration
When CyA was initiated the previous dose of corticosteroids was not changed. CyA was administered intravenously at a dose of 4 mg/kg/day in two daily doses given in 250 ml of 5% glucosate fluid for 4 hours (28). The dose was adjusted according to CyA serum levels at 48 hours (normal CyA levels: 100-400 ng/ml, determined by RIA). In case of good response, defined as a decrease in mTLW of at least 10 points, CyA was changed to oral administration (5 mg/kg/day) for three months (29). Serum CyA levels were monitored weekly during admission and monthly during follow-up in the outpatient clinic. Oral azathioprine (2-2.5 mg/kg/day) was added, and lab controls were done periodically.
Collection of disease related variables
In all patients data collected included demographic variables (age, sex, smoking habit (nonsmoker, smoker, former smoker), family history of inflammatory bowel disease). Disease characteristics: extension - pancolitis vs. left-sided colitis (30), duration in months, number and severity of previous flares, number of previous admissions, number of blood transfusions, need of corticosteroids, immunosupressants, antibiotics, and CyA in previous flares). Characteristics of present relapse: temperature, heart rate, number of bowel movements, abdominal pain severity, rectal syndrome, presence of deep ulcers during sigmoidoscopy, mTLW, extraintestinal manifestations, number of blood transfusions, body weight, laboratory - hemoglobin, leukocytes, non-segmented neutrophils, globular sedimentation rate, fibrinogen, C-reactive protein, fibrinogen, platelet count, albumin and fecal Clostridium difficile toxin at CyA onset.
The complications of CyA were also recorded as minor or major (31). For major complications CyA was withdrawn, and for minor complications CyA dosage was adjusted.
Response criteria
According to clinical response to CyA at day 10, three groups of patients were defined: absence of response to treatment, defined as lack of clinical improvement (decrease of 5 or fewer points in mTLW after 10 days of treatment). Partial response, defined as a decrease in mTLW score > 5 points but without reaching remission. Complete response, defined as clinical remission (mTLM score lower than 11) after 10 days of treatment.
Statistical analysis
Clinical cards were exported to an Excel workbook. Statistical analyses were performed by using the statistics program GraphPad InStat. To compare qualitative variables the exact Fischer's test was used. To compare quantitative variables the Mann-Whitney test was used. Statistically significant differences were considered when p < 0.05. Variables statistically different between responsive and non-responsive groups in the univariate analysis were included in a multiple linear regression analysis to identify independent predictive factors of poor response to treatment with CyA.
Results
The clinical characteristics of patients are shown in table I. Thirty-five patients with severe UC refractory to steroids who received cyclosporine intravenously were included. Six patients also received CyA previously in other flare-ups. Thus, a total of 41 flares were studied. Fifteen patients (42.8%) were female. Median age was 34 (26-40) years at admission. Five patients (14.3%) were smokers and 21 (60%) were non-smokers. Eight patients were on maintenance treatment with azathioprine (22.9%).
A complete response to CyA after 10 days of treatment was achieved in 20/41 flares (48.8%), a partial response in 9 (22%), and absence of response in 12 (29.2%) (Table II).
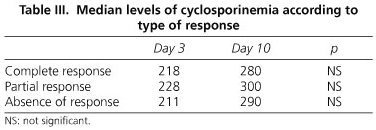
There were no statistical differences between groups regarding type of response to CyA and serum CyA at days 3 or 10 (Table III).
Factors associated with absence of clinical response
To determine which epidemiological, clinical or biological parameters could shortly predict absence of response to intravenous CyA, different variables were used (Table IV). A univariate analysis between patients with complete response and absence of response was performed (Table V) to identify subjects to be included in a subsequent multivariate analysis.
Patients with complete response were younger than those with absence of response (30 (24-40) years vs. 40 (34-42), p < 0.001). Sex, smoking, maintenance treatment, and disease duration before inclusion did not influence response to intravenous CyA. On the other hand, colitis extent (pancolitis vs. left-sided colitis), thrombocytosis, and a history of previous treatment with intravenous CyA in other flares were significantly associated (p < 0.05) with absence of response to CyA.
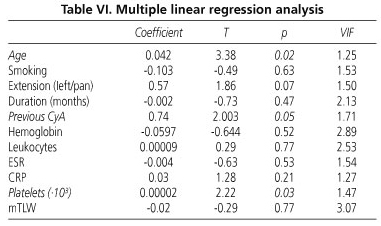
Table VI shows the results of the multiple linear regression test. Independent predictive variables of short absence of response to CyA in severe UC refractory to corticosteroids included age (p = 0.02), previous intravenous CyA (p = 0.05), and platelet count (p = 0.03). According to these results, older patients with a history of previous treatment with CyA and thrombocytosis during the present flare-up would be the poorest responders to CyA.
Discussion
There is important evidence supporting the efficacy of CyA in the treatment of UC refractory to corticosteroids, with a range of responses between 60 and 85% (7,32-36). In our study, clinical remission was achieved in about half of patients, a result that we consider excellent as it was determined after only 10 days of treatment. Other studies analyzing the results of CyA in refractory UC after two weeks or three months of treatment have described better results, suggesting that length of treatment with CyA is important in determining efficacy.
Several studies have identified predictive factors for lack of response to intravenous corticosteroids: severe endoscopic lesions, C-reactive protein higher than 45 mg/L after 3 days of intensive treatment, more than 8 bowel movements per day, toxic megacolon, low albumin levels, and long duration of present flare (20,37-39). However, there is scarce information on predictive factors for response to CyA. Our study has identified three predictors of poor response to CyA, one biological (thrombocytosis) and 2 epidemiological (age and history of previous treatment with CyA) in nature.
Rowe et al. (40), in a retrospective study of 36 patients, identified three predictive factors of absence of response to CyA: tachycardia, hypoalbuminemia, and increase in non-segmented neutrophils. More recently two studies have been reported that looked at predictive factors for response to CyA. Cacheux et al. (41) identified three variables (tachycardia, fever, and C-reactive protein higher than 45 mg/dl) as predictive factors for colectomy. Aceituno et al. (42) revealed an association of need for colectomy with increased levels of C-reactive protein.
The lack of consistency between predictors identified in our study and those published by other studies may be due to the fact that we decided to assess response to treatment after only 10 days, and due to study design limitations (retrospective study, no colonoscopic controls) (43). Colonoscopy was not performed in all patients at inclusion because of the risk for complications in these subjects. Consequently, our study does not include colonic mucosa appearance as a potential predictive factor.
In conclusion, three predictors of absence of response to CyA in UC patients refractory to corticosteroids have bee identified: thrombocytosis, older age, and a history of previous treatment with CyA. In these cases other therapeutic alternatives have to be considered, although no guidelines may be suggested from this study.
References
1. Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology 2007; 133: 1670-89. [ Links ]
2. Loftus E. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004; 126: 1504-17. [ Links ]
3. Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. Gut 1963; 41: 299-315. [ Links ]
4. Abu-Suboh M, Casellas F, Vilaseca J, Malagelada JR. Response of first attack of inflammatory bowel disease requiring hospital admission to steroid therapy. Rev Esp Enferm Dig 2004; 96: 539-47. [ Links ]
5. Benazzato L, D'Incà R, Grigoletto F, Perissinotto E, Medici V, Angriman I, et al. Prognosis of severe attacks in ulcerative colitis: effect of intensive medical treatment. Dig Liver Dis 2004; 36: 461-6. [ Links ]
6. Truelove SC, Jewell DP. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet 1974; 1: 1067-70. [ Links ]
7. Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994; 330: 1841-5. [ Links ]
8. Carbonnel F, Boruchowicz A, Duclos B, Soulé JC, Lerebours E, Lémann M, et al. Intravenous cyclosporine in attacks of ulcerative colitis: short-term and long-term responses. Dig Dis Sci 1996; 41: 2471-6. [ Links ]
9. Conor G, Laurence J. Cyclosporine, tacrolimus, and mycophenolate mofetil in the treatment of inflammatory bowel disease. Gastroenterol Clin North Am 2004; 33: 141-69. [ Links ]
10. Sandborn WJ. Cyclosporine therapy for inflammatory bowel disease: definitive answer and remaining questions. Gastroenterology 1995; 109: 1001-3. [ Links ]
11. Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, Grännö C, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: A randomized, placebo-controlled study. Gastroenterology 2005; 128: 1805-11. [ Links ]
12. Rutgeers P, Sandborn W, Reinisch W, Olson A, Johanns J, Travers S, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462-76. [ Links ]
13. Ferrante M, Vermeire S, Katsanos KH, Noman M, Van Assche G, Schnitzler F, et al. Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm Bowel Dis 2007; 13: 123-8. [ Links ]
14. Aberra F, Lichtenstein G. Infliximab in ulcerative colitis. Gastroenterol Clin North Am 2006; 33: 821-36. [ Links ]
15. Rutgeerts P, Van Assche G. Review article: infliximab therapy for inflammation bowel disease-seven years on. Aliment Pharmacol Ther 2006; 23: 451-63. [ Links ]
16. Willert R, Lawrance I. Use of infliximab in the prevention and delay of colectomy in severe steroid dependant and refractory ulcerative colitis. World J Gastroenterol 2008; 14: 2544-9. [ Links ]
17. Ricart E, Esteve M, Andreu M, Casellas F, Monfort D, Sans M, et al. Evaluation of 5 versus 10 granulocyteaphaeresis sessions in steroid-dependent ulcerative colitis: A pilot, prospective, multicenter, randomized study. World J Gastroenterol 2007; 13: 2193-7. [ Links ]
18. Susuki Y, Yoshimura N, Fukuda K, Shirai K, Saito Y, Saniabadi AR. A retrospective search for predictors of clinical response to selective granulocyte and monocyte apheresis in patients with ulcerative colitis. Dig Dis Sci 2006; 51: 2031-8. [ Links ]
19. Takemoto K, Kato J, Kuriyama M, Nawa T, Kurome M, Okada H, et al. Predictive factors of efficacy of leukocytapheresis for steroid-resistant ulcerative colitis patients. Dig Liver Dis 2007; 39: 422-9. [ Links ]
20. Travis SPL, Farrant JM, Ricketts C, Nolan DJ, Mortensen NM, Ke-ttlewell MG, et al. Predicting outcome in severe ulcerative colitis. Gut 1996; 38: 905-10. [ Links ]
21. Bernal I, Mañosa M, Domenech E, García-Planella, Navarro M, Cabré E, et al. Predictors of clinical response to systemic steroids in active ulcerative colitis. Dig Dis Sci 2006; 51: 1434-8. [ Links ]
22. Carbonnel F, Gargouri D, Lémann M, Beaugerie L, Cattan S, Cosnes J, et al. Predictive factors of outcome of intensive intravenous treatment for attacks of ulcerative colitis. Aliment Pharmacol Ther 2000; 14: 273-8. [ Links ]
23. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl 1989; 170: 2-6. [ Links ]
24. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology Practice and Parameters committee. Am J Gastroenterol 2004; 99: 1371-85. [ Links ]
25. Stange EF. European evidence-based Consensus on the diagnosis and management of ulcerative colitis. J Crohn Colitis 2008; 2: 1-62. [ Links ]
26. Truelove SC, Witts LJ. Cortisone in ulcerative colitis: final report on a therapeutic trial. Br Med J 1955; 2: 1041-8. [ Links ]
27. D'Haens, Sandborn WJ, Feagan BG, K, Hanauer SB, Irvine EJ, Lémann M, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007; 132: 763-86. [ Links ]
28. Van AG, D`Haens G, Noman M Vermeire S, Hiele M, Asnong K, et al. Randomized double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 2003; 125: 1025-31. [ Links ]
29. Weber A, Fein F, Koch S, Dupont-Gossart AC, Mantion G, Heyd B, et al. Treatment of ulcerative colitis refractory to steroid therapy by oral microemulsion cyclosporine (Neoral). Inflamm Bowel Dis 2006; 12: 1131-5. [ Links ]
30. Silverberg MS, Sattsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Supl. A): 5A-36A. [ Links ]
31. Stein R, Cohen R, Hanauer S. Complications during cyclosporine therapy for inflammatory bowel disease. Gastroenterology 1997; 112: A1096. [ Links ]
32. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporine in ulcerative colitis: A five-year experience. Am J Gastroenterol 1999; 94: 1587-92. [ Links ]
33. Santos J, Baudet S, Casellas F, Guarner L, Vilaseca J, Malagelada JR. Efficacy of intravenous cyclosporine for steroid refractory of ulcerative colitis. J Clin Gastroenterol 1995; 90: 2093-6. [ Links ]
34. Message L, Bourrelle A, Lahaire D, Quinton A, Galmiche JP, Lamouliatte H, et al. Efficacy of intravenous cyclosporine in moderately severe ulcerative colitis refractory to steroids. Gastroenterol Clin Biol 2005; 29: 231-5. [ Links ]
35. Sands B. New Therapies for the treatment of inflammatory bowel disease. Surg Clin North Am 2006; 86: 1045-64. [ Links ]
36. Actis G, Fadda M, David E, Sapino A. Colectomy rate in steroid-refractory colitis initially responsive to cyclosporine: a long-term retrospective cohort study. BMC Gastroenterology 2007; 7: 1-6. [ Links ]
37. Ho GT, Mowat C, Goddard CJ, Fennell JM, Shah NB, Prescott RJ, Satsangi J. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther 2004; 19: 1079-87. [ Links ]
38. Solem CA, Loftus Jr EV, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis 2005; 11: 707-12. [ Links ]
39. Chang J, Cohen R. Medical management of severe ulcerative colitis. Gastroenterol Clin North Am 2004; 33: 235-50. [ Links ]
40. Rowe FA, Walker JH, Karp LC, Vasiliauskas EA, Plevy SE, Targan SR. Factors predictive of response to cyclosporine treatment for severe, steroid-resistant ulcerative colitis. Am J Gastroenterol 2000; 95: 2000-8. [ Links ]
41. Cacheux W, Seksik P, Lemmann M, Marteau P, Nion-Larmurier I, Afchain P, et al. Predictive factors of response to cyclosporine in steroid-refractory ulcerative colitis. Am J Gastroenterol 2008; 103: 637-42. [ Links ]
42. Aceituno M, García-Planella E, Heredia C, Zabana Y, Feu F, Domènech E, et al. Steroid-refractory ulcerative colitis: Predictive factors of response to cyclosporine and validation in an independent cohort. Inflamm Bowel Dis 2008; 14: 347-52. [ Links ]
43. Ando T, Nishio Y, Watanabe O, Maeda O, Ishiguro K, Ishikawa D, et al. Value of colonoscopy for prediction of prognosis in patients with ulcerative colitis. World J Gastroenterol 2008; 14: 2133-8. [ Links ]
![]() Correspondence:
Correspondence:
Francesc Casellas.
Unitat d'Atenció Crohn-Colitis.
Servicio de Digestivo.
Hospital Universitari Vall d'Hebron.
Pso. Vall d'Hebron, 119.
08035 Barcelona, Spain.
e-mail: fcasella@vhebron.net
Received: 12-01-09.
Accepted: 15-01-09.











 texto en
texto en